Abstract
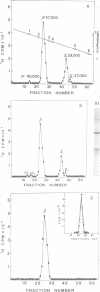
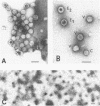
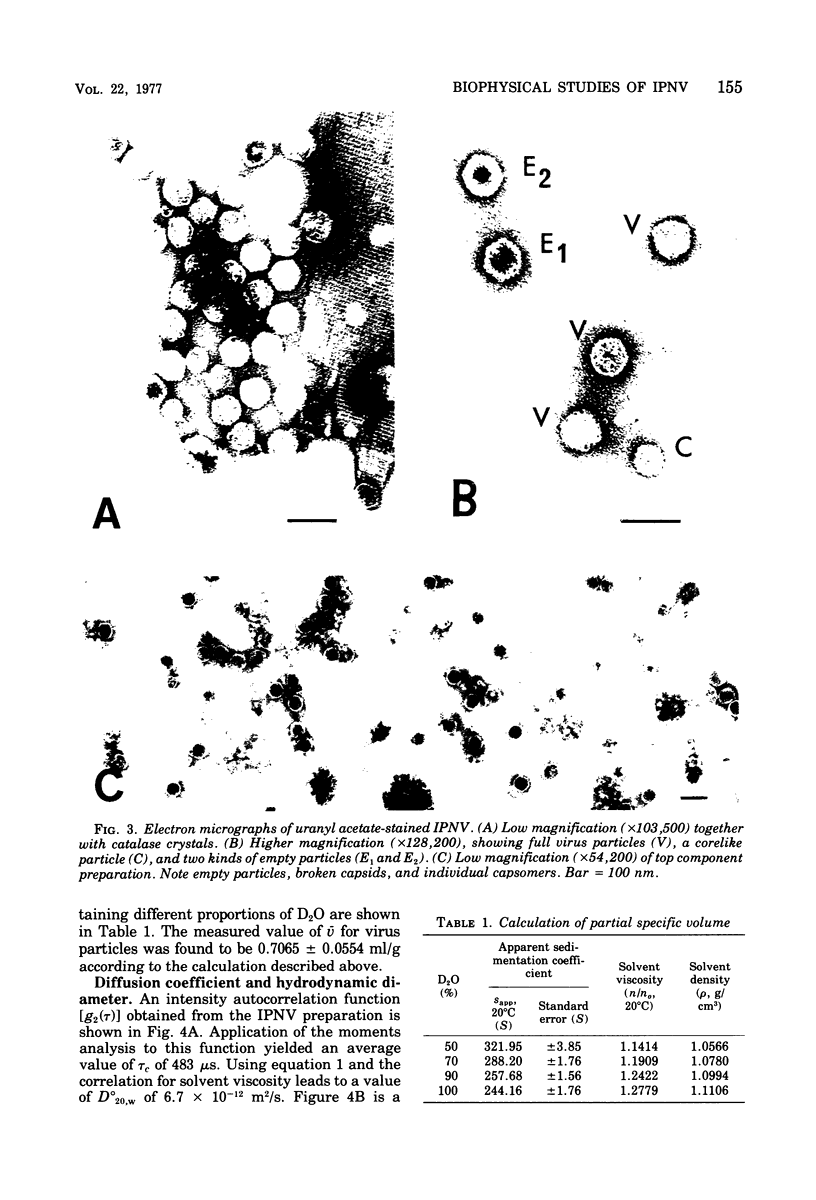
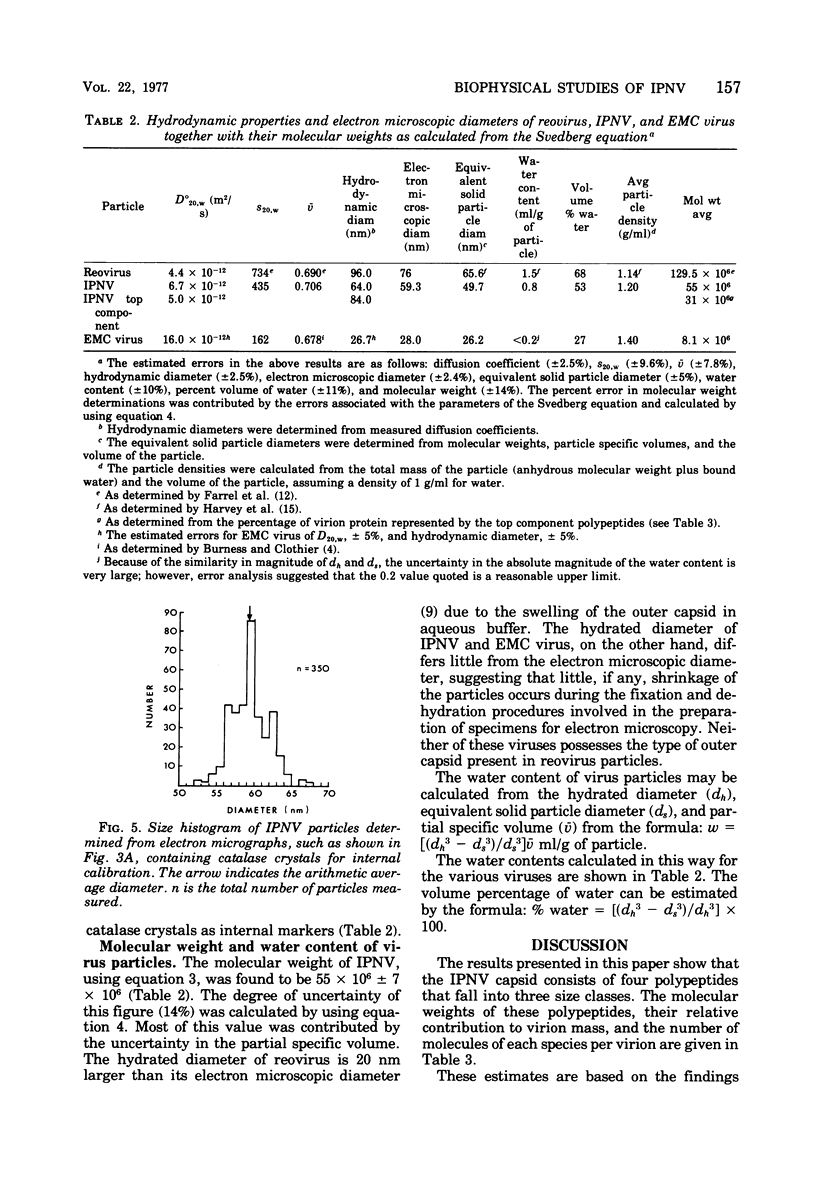
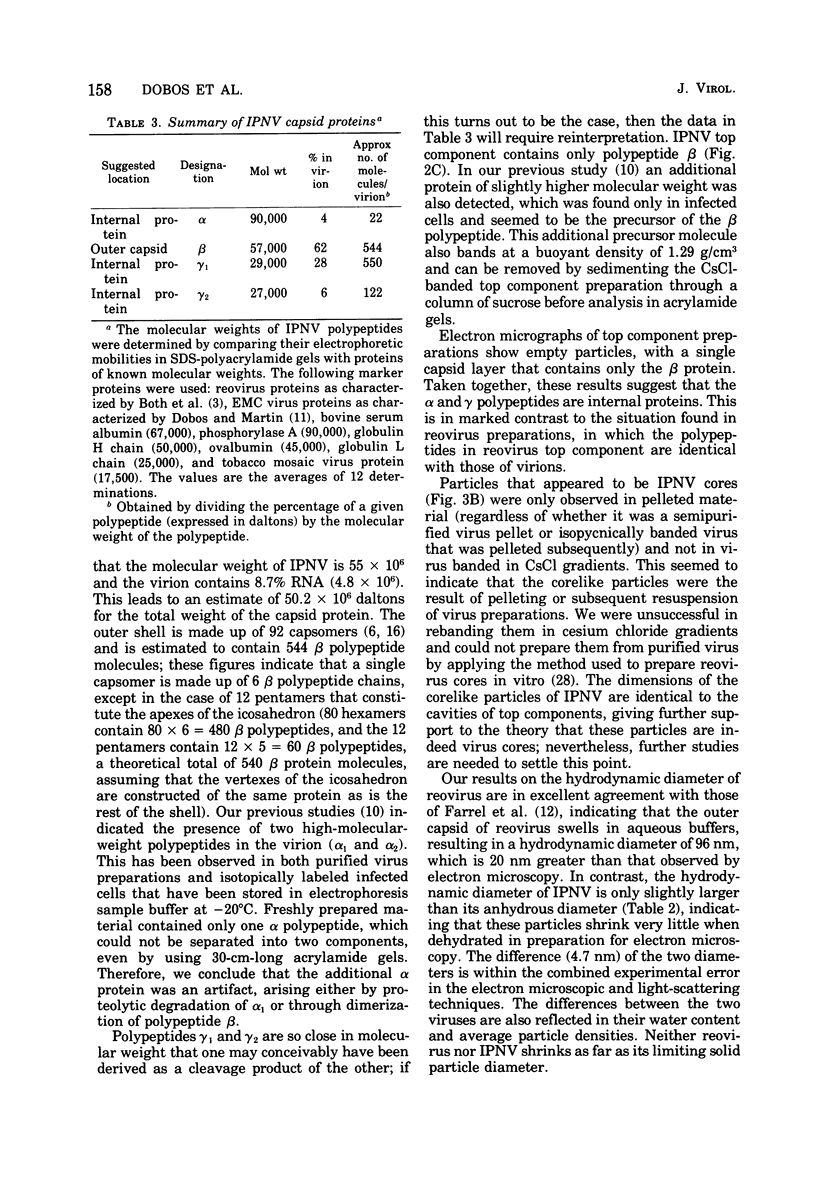
The molecular weight of infectious pancreatic necrosis virus (IPNV) has been determined by analytical ultracentrifugation and dynamic light scattering. The sedimentation coefficient of the virus was found to be 435S. The average value for molecular weight is (55 +/- 7) x 106. The virus genome consists of two segments of double-stranded RNA (molecular weights, 2.5 x 106 and 2.3 x 106), which represents 8.7% of the virion mass. The capsid protein moiety of IPNV consists of four species of polypeptides, as determined by polyacrylamide gel electrophoresis. The number of molecules of each polypeptide in the virion has been determined. There are 22 molecules of the internal polypeptide alpha (molecular weight, 90,000), 544 molecules of the outer capsid polypeptide beta (molecular weight, 57,000), and 550 and 122 molecules, respectively, of the internal polypeptides gamma1 (molecular weight, 29,000) and gamma2 (molecular weight, 27,000). IPNV top component contains only the beta polypeptide species, and its molecular weight is estimated to be 31 x 106. The hydrodynamic diameter and electron microscopic diameter (calculated by catalase crystal-calibrated electron microscopy) of IPNV was compared with those of reovirus and encephalomyocarditis virus. Due to the swelling of the outer capsid, reovirus particles were found to be much larger when hydrated (96-nm diameter) than when dehydrated (76-nm diameter), having a large water content content and low average density. In contrast, IPNV particles are more rigid, having nearly the same average diameter under hydrous (64 nm) as under anhydrous conditions (59.3 nm). Encephalomyocarditis virus has a very low water content and does not shrink at all when prepared for electron microscopy.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Both G. W., Lavi S., Shatkin A. J. Synthesis of all the gene products of the reovirus genome in vivo and in vitro. Cell. 1975 Feb;4(2):173–180. doi: 10.1016/0092-8674(75)90124-5. [DOI] [PubMed] [Google Scholar]
- Burness A. T., Clothier F. W. Particle weight and other biophysical properties of encephalomyocarditis virus. J Gen Virol. 1970 Mar;6(3):381–393. doi: 10.1099/0022-1317-6-3-381. [DOI] [PubMed] [Google Scholar]
- Camerini-Otero R. D., Pusey P. N., Koppel D. E., Schaefer D. W., Franklin R. M. Intensity fluctuation spectroscopy of laser light scattered by solutions of spherical viruses: R17, Q beta, BSV, PM2, and T7. II. Diffusion coefficients, molecular weights, solvation, and particle dimensions. Biochemistry. 1974 Feb 26;13(5):960–970. doi: 10.1021/bi00702a021. [DOI] [PubMed] [Google Scholar]
- Cohen J., Poinsard A., Scherrer R. Physico-chemical and morphological features of infectious pancreatic necrosis virus. J Gen Virol. 1973 Dec;21(3):485–498. doi: 10.1099/0022-1317-21-3-485. [DOI] [PubMed] [Google Scholar]
- Dobos P., Martin E. M. Virus-specific polypeptides in ascites cells infected with encephalomyocarditis virus. J Gen Virol. 1972 Nov;17(2):197–212. doi: 10.1099/0022-1317-17-2-197. [DOI] [PubMed] [Google Scholar]
- Dobos P. Size and structure of the genome of infectious pancreatic necrosis virus. Nucleic Acids Res. 1976 Aug;3(8):1903–1924. doi: 10.1093/nar/3.8.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobos P. Use of gum tragacanth overlay, applied at room temperature, in the plaque assay of fish and other animal viruses. J Clin Microbiol. 1976 Mar;3(3):373–375. doi: 10.1128/jcm.3.3.373-375.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobos P. Virus-specific protein synthesis in cells infected by infectious pancreatic necrosis virus. J Virol. 1977 Jan;21(1):242–258. doi: 10.1128/jvi.21.1.242-258.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell J. A., Harvey J. D., Bellamy A. R. Biophysical studies of reovirus type 3. Virology. 1974 Nov;62(1):145–153. doi: 10.1016/0042-6822(74)90310-9. [DOI] [PubMed] [Google Scholar]
- Gomatos P. J., Tamm I. THE SECONDARY STRUCTURE OF REOVIRUS RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):707–714. doi: 10.1073/pnas.49.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J. D., Farrell J. A., Bellamy A. R. Biophysical studies of reovirus type 3. II. Properties of the hydrated particle. Virology. 1974 Nov;62(1):154–160. doi: 10.1016/0042-6822(74)90311-0. [DOI] [PubMed] [Google Scholar]
- Kelly R. K., Loh P. C. Electron microscopical and biochemical characterization of infectious pancreatic necrosis virus. J Virol. 1972 Oct;10(4):824–834. doi: 10.1128/jvi.10.4.824-834.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M., Martin E. M. Simple method for the isolation of encephalomyocarditis virus ribonucleic acid. J Virol. 1972 Mar;9(3):559–561. doi: 10.1128/jvi.9.3.559-561.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Malsberger R. G., Cerini C. P. Multiplication of infectious pancreatic necrosis virus. Ann N Y Acad Sci. 1965 Aug 10;126(1):320–327. doi: 10.1111/j.1749-6632.1965.tb14283.x. [DOI] [PubMed] [Google Scholar]
- Reddy D. V., Black L. M. Electrophoretic separation of all components of the double-stranded RNA of wound tumor virus. Virology. 1973 Aug;54(2):557–562. doi: 10.1016/0042-6822(73)90168-2. [DOI] [PubMed] [Google Scholar]
- Salmeen I., Rimai L., Liebes L., Rich M. A., McCormick J. J. Hydrodynamic diameters of RNA tumor viruses. Studies by laser beat frequency light scattering spectroscopy of avian myeloblastosis and Rauscher murine leukemia viruses. Biochemistry. 1975 Jan 14;14(1):134–141. doi: 10.1021/bi00672a023. [DOI] [PubMed] [Google Scholar]
- Salmeen I., Rimai L., Luftig R. B., Libes L., Retzel E., Rich M., McCormick J. J. Hydrodynamic diameters of murine mammary, Rous sarcoma, and feline leukemia RNA tumor viruses: studies by laser beat frequency light-scattering spectroscopy and electron microscopy. J Virol. 1976 Feb;17(2):584–596. doi: 10.1128/jvi.17.2.584-596.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. E., Zweerink H. J., Joklik W. K. Polypeptide components of virions, top component and cores of reovirus type 3. Virology. 1969 Dec;39(4):791–810. doi: 10.1016/0042-6822(69)90017-8. [DOI] [PubMed] [Google Scholar]
- Ware B. R., Raj T., Flygare W. H., Lesnaw J. A., Reichmann M. E. Molecular weights of vesicular stomatitis virus and its defective particles by laser light-scattering spectroscopy. J Virol. 1973 Jan;11(1):141–145. doi: 10.1128/jvi.11.1.141-145.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrigley N. G. The lattice spacing of crystalline catalase as an internal standard of length in electron microscopy. J Ultrastruct Res. 1968 Sep;24(5):454–464. doi: 10.1016/s0022-5320(68)80048-6. [DOI] [PubMed] [Google Scholar]