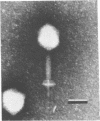
Abstract
A pseudolysogenic phage, PMB1, was isolated from soil on the basis of its ability to increase the sporulation frequency of the oligosporogenic Bacillus pumilus strain NRS 576 (sporulation frequency, less than 1%). Several spore-negative mutants (sporulation frequency, less than 10-8) derived from strain NRS 576, which were converted to spore positive by infection with PMB1, were subsequently identified. PMB1 repeatedly grown on a given spore-negative mutant (e.g., GW2) converted GW2 cells to spore positive. Each plaque-forming unit initiated the conversion of a spore-positive clone in semisolid agar overlays. GW2 cells remained spore positive as long as they maintained PMB1. Return of PMB1-converted cells to the orginal spore-negative phenotype correlated with loss of PMB1. In liquid media, PMB1 infection increased the sporulation frequency of mutant GW2 over 106-fold. More than half of the spore-negative mutants we isolated from strain NRS 576 were converted to spore positive by PMB1 infection. PMB1-induced spores of the spore-negative mutant GW2 were somewhat more heat sensitive than uninfected or PMB1-infected spores of the spore positive parent of GW2. PMB1-induced spores of GW2 do not differ from wild-type spores in morphology by phase-contrast microscopy, dipicolinic acid content, or rate of sedimentation through Renografin gradients.
Full text
PDF


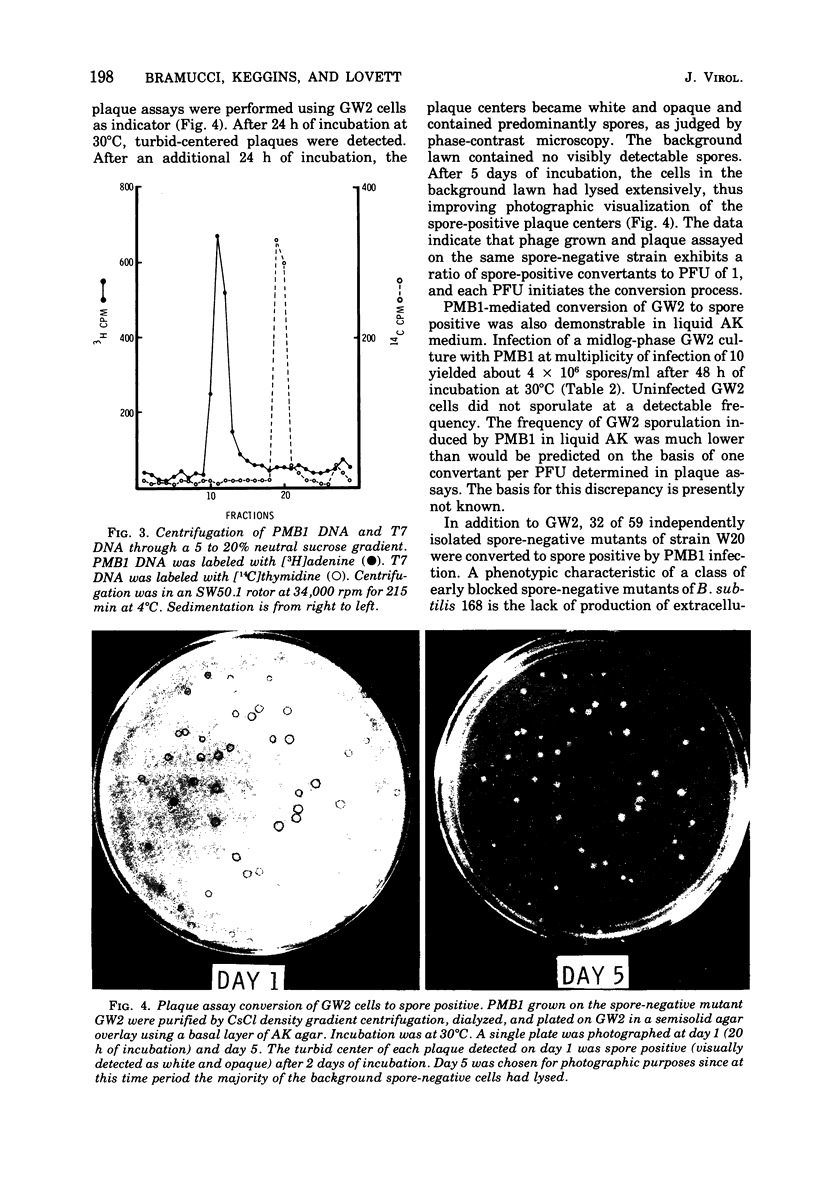
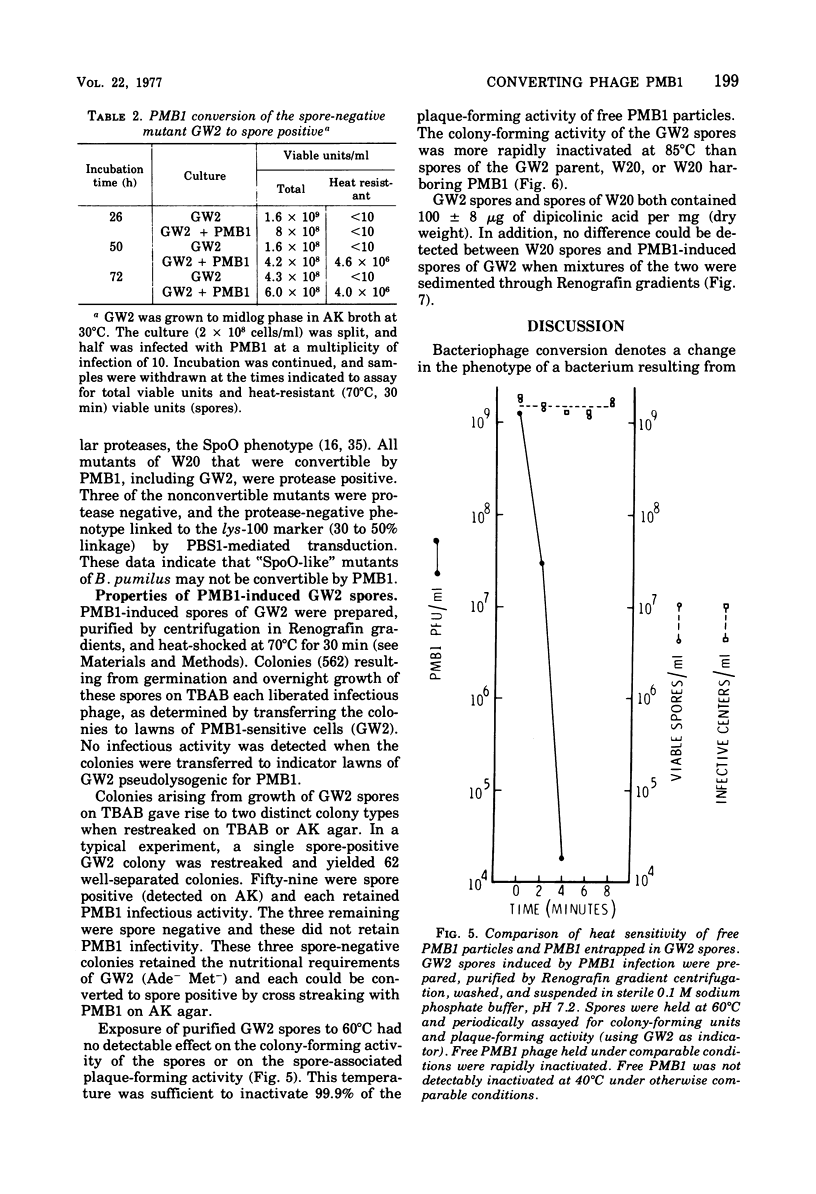


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barksdale L., Arden S. B. Persisting bacteriophage infections, lysogeny, and phage conversions. Annu Rev Microbiol. 1974;28(0):265–299. doi: 10.1146/annurev.mi.28.100174.001405. [DOI] [PubMed] [Google Scholar]
- Bramucci M. G., Lovett P. S. Low-frequency, pbsi-mediated plasmid transduction in Bacillus pumilus. J Bacteriol. 1976 Aug;127(2):829–831. doi: 10.1128/jb.127.2.829-831.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramucci M. G., Lovett P. S. Temperate bacteriophage infectious for asporogenic variants of Bacillus pumilus. J Virol. 1974 Nov;14(5):1281–1287. doi: 10.1128/jvi.14.5.1281-1287.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. H., Orrego J. C., Hutchison K. W., Halvorson H. O. New temperate bacteriophage for Bacillus subtilis, rho 11. J Virol. 1976 Nov;20(2):509–519. doi: 10.1128/jvi.20.2.509-519.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denney R. M., Yanofsky C. Isolation and characterization of specialized phi80 transducing phages carrying regions of the Salmonella typhimurium trp operon. J Bacteriol. 1974 May;118(2):505–513. doi: 10.1128/jb.118.2.505-513.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich S. D., Bursztyn-Pettegrew H., Stroynowski I., Lederberg J. Expression of the thymidylate synthetase gene of the Bacillus subtilis bacteriophage Phi-3-T in Escherichia coli. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4145–4149. doi: 10.1073/pnas.73.11.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund M. W., Poysky F. T., Meyers J. A., Pelroy G. A. Interspecies conversion of Clostridium botulinum type C to Clostridium novyi type A by bacteriophage. Science. 1974 Nov 1;186(4162):456–458. doi: 10.1126/science.186.4162.456. [DOI] [PubMed] [Google Scholar]
- FREEMAN V. J. Studies on the virulence of bacteriophage-infected strains of Corynebacterium diphtheriae. J Bacteriol. 1951 Jun;61(6):675–688. doi: 10.1128/jb.61.6.675-688.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garro A. J., Law M. F. Relationship between lysogeny, spontaneous induction, and transformation efficiencies in Bacillus subtilis. J Bacteriol. 1974 Dec;120(3):1256–1259. doi: 10.1128/jb.120.3.1256-1259.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadden C., Nester E. W. Purification of competent cells in the Bacillus subtilis transformation system. J Bacteriol. 1968 Mar;95(3):876–885. doi: 10.1128/jb.95.3.876-885.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemphill H. E., Whiteley H. R. Bacteriophages of Bacillus subtilis. Bacteriol Rev. 1975 Sep;39(3):257–315. doi: 10.1128/br.39.3.257-315.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higerd T. B., Hoch J. A., Spizizen J. Hyperprotease-producing mutants of Bacillus subtilis. J Bacteriol. 1972 Nov;112(2):1026–1028. doi: 10.1128/jb.112.2.1026-1028.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A. Genetics of bacterial sporulation. Adv Genet. 1976;18:69–98. doi: 10.1016/s0065-2660(08)60437-x. [DOI] [PubMed] [Google Scholar]
- Holmes R. K., Barksdale L. Genetic analysis of tox+ and tox- bacteriophages of Corynebacterium diphtheriae. J Virol. 1969 Jun;3(6):586–598. doi: 10.1128/jvi.3.6.586-598.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Spizizen J. Increased rate of asporogenous mutations following treatment of Bacillus subtilis spores with ethyl methanesulfonate. Mutat Res. 1971 Sep;13(1):93–96. doi: 10.1016/0027-5107(71)90130-8. [DOI] [PubMed] [Google Scholar]
- Losick R., Sonenshein A. L. Change in the template specificity of RNA polymerase during sporulation of Bacillus subtilis. Nature. 1969 Oct 4;224(5214):35–37. doi: 10.1038/224035a0. [DOI] [PubMed] [Google Scholar]
- Lovett P. S., Bramucci M. G. Biochemical studies of two Bacillus pumilus plasmids. J Bacteriol. 1974 Oct;120(1):488–494. doi: 10.1128/jb.120.1.488-494.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S., Duvall E. J., Keggins K. M. Bacillus pumilus plasmid pPL10: properties and insertion into Bacillus subtilis 168 by transformation. J Bacteriol. 1976 Aug;127(2):817–828. doi: 10.1128/jb.127.2.817-828.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S. PBPI: a flagella specific bacteriophage mediating transduction in Bacillus pumilus. Virology. 1972 Mar;47(3):743–752. doi: 10.1016/0042-6822(72)90564-8. [DOI] [PubMed] [Google Scholar]
- Lovett P. S. Plasmid in Bacillus pumilus and the enhanced sporulation of plasmid-negative variants. J Bacteriol. 1973 Jul;115(1):291–298. doi: 10.1128/jb.115.1.291-298.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S., Young F. E. Genetic analysis in Bacillus pumilus by PBSI-mediated transduction. J Bacteriol. 1970 Feb;101(2):603–608. doi: 10.1128/jb.101.2.603-608.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBINS P. W., UCHIDA T. Studies on the chemical basis of the phage conversion of O-antigens in the E-group Salmonellae. Biochemistry. 1962 Mar;1:323–335. doi: 10.1021/bi00908a020. [DOI] [PubMed] [Google Scholar]
- Rogolsky M. The mapping of genes for spore formation on the chromosome of Bacillus licheniformis. Can J Microbiol. 1970 Jul;16(7):595–600. doi: 10.1139/m70-099. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHASHI I. INCORPORATION OF BACTERIOPHAGE GENOME BY SPORES OF BACILLUS SUBTILIS. J Bacteriol. 1964 Jun;87:1499–1502. doi: 10.1128/jb.87.6.1499-1502.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker R. G. Acquisition of thymidylate synthetase activity by a thymine-requiring mutant of Bacillus subtilis following infection by the temperate phage phi 3. J Gen Virol. 1969 Jun;4(4):489–504. doi: 10.1099/0022-1317-4-4-489. [DOI] [PubMed] [Google Scholar]
- Wilson G. A., Williams M. T., Baney H. W., Young F. E. Characterization of temperate bacteriophages of Bacillus subtilis by the restriction endonuclease EcoRI: evidence for three different temperate bacteriophages. J Virol. 1974 Oct;14(4):1013–1016. doi: 10.1128/jvi.14.4.1013-1016.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasbin R. E., Wilson G. A., Young F. E. Transformation and transfection in lysogenic strains of Bacillus subtilis 168. J Bacteriol. 1973 Feb;113(2):540–548. doi: 10.1128/jb.113.2.540-548.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]