Figure 1.
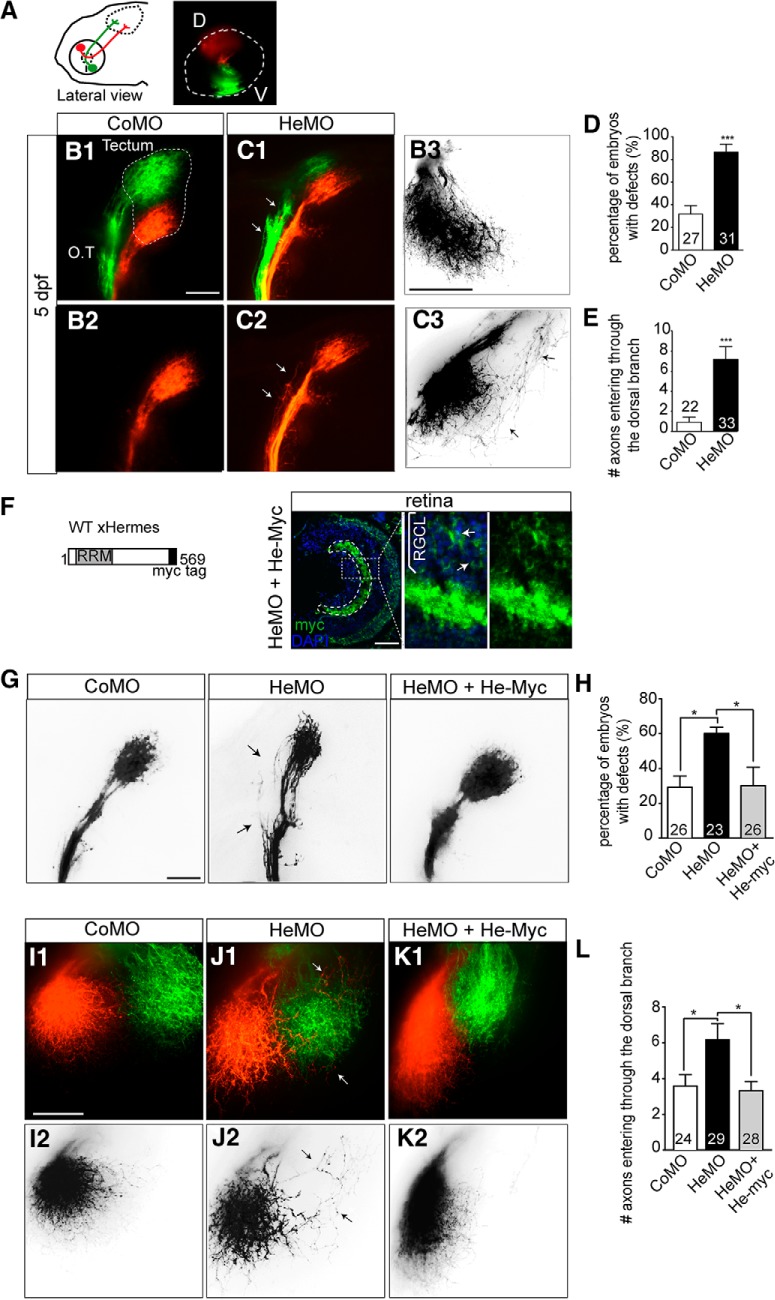
Knock-down of Hermes causes topographic guidance defects of RGC dorsal axons. Zebrafish embryos were fixed at 5 dpf and the eyes injected dorsally (D) with DiI (red) and ventrally (V) with DiO (green) to visualize the retinotectal projections (A). Whole-mount embryos injected with CoMO or HeMO were visualized in lateral (B1, B2, C1, C2) or dorsal view (B3, C3). Hermes-depleted embryos show misprojections of dorsal axons in the medial tract (C1, C2, white arrows) that are not present in embryos injected with CoMO (B1, B2). Quantifications show a significant increase of the percentage of embryos showing misprojections in the OT (D). HeMO-injected embryos show aberrant projection of dorsal axons entering the tectum through the ventral branch (C3) compared with CoMO (B3). Quantifications show that significantly more dorsal axons misroute and enter the tectum through the ventral branch in Hermes-depleted embryos compared with control (E). HeMO-injected embryos were coinjected with a construct expressing full-length myc-tagged Xenopus Hermes (He-myc; F). After 72 hpf, immunostainings show a strong myc signal in the RGC layer (RGCL). Coinjection of He-myc rescues the dorsal axons misprojections in the OT observed in HeMO-injected embryos (G), with a significant reduction of the percentage of embryos with defects (H). In HeMO-injected embryos, some dorsal axons enter in the tectum through the medial tectum (J1, J2) and this mistargeting is absent in He-myc-coinjected embryos (K1, K2). Quantifications show a rescue of misprojecting dorsal axons in He-myc-injected embryos (L). Error bars indicate SEM. Numbers of embryos analyzed are indicated on bars. Scale bars, 50 μm.