This editorial refers to ‘C-type natriuretic peptide and natriuretic peptide receptor NPR-B signalling inhibits cardiac sympathetic neurotransmission and autonomic function’ by J. Buttgereit et al., pp. 637–644.
Natriuretic peptides (NPs) are a family of secretory peptides that have in common a ringed amino-acid structure linked by disulphide bonds1 which signal via NP receptors (NPRs). Three primary members have been identified: atrial NP (ANP), brain-derived NP (BNP), and C-type NP (CNP), but there is a closely related forth peptide Dendroaspis NP (DNP). ANP and BNP are elevated by atrial stretch; ventricular stretch or stress elevates BNP levels; and all NPs are increased in heart failure (HF). The NPRs are particulate guanylyl-cyclase-coupled receptors which stimulate production of cGMP. Of the seven NPR isoforms known to exist, the majority of NP physiological actions are mediated via NPR-A and -B, while NPR-C mediates NP degradation. ANP, BNP, and DNP activate NP receptor A (NPR-A), which increases natriuresis and vasodilation. In contrast, CNP specifically activates NPR-B and does not possess the potent diuretic actions of the other NPs.
Although extensive studies have been performed on BNP in the context of HF, where it has been utilized both as a diagnostic and as a potential therapeutic tool,2 newer data implicate the peptide in modulating peripheral autonomic neurotransmission. BNP activation of NPR-A inhibits neurotransmission from sympathetic neurons by decreasing the magnitude of intracellular calcium transients, and impairing neurotransmitter release.3 Phosphodiesterase-2A (PDE2A) levels act as an intracellular switch in this process permitting or preventing the effect of BNP on neurotransmission. CNP, which is present in endothelial cells and ventricular myocardium, is also abundant in the nervous system. CNP levels increase in HF, similar to ANP and BNP, but its physiological relevance has not been well understood.
In this issue of the journal Buttgereit et al.4 extend our understanding of the physiological roles played by CNP, using a rat model overexpressing a neuron-specific dominant negative form of NPR-B. The authors show that inhibition of NPR-B signalling results in sympathoexcitation, analogous to the physiological effect of blocking NPR-A signalling. The heightened sympathetic transmission causes hypertension, tachycardia, and a decrease in heart rate variability. Rats with impaired NPR-B signalling also develop left ventricular dysfunction, likely mediated by excessive sympathoexcitation, analogous to what is clinically observed as Tako-Tsubo syndrome. The authors show in an ex vivo prep that CNP decreases the tachycardia response to nerve stimulation, but not to direct norepinephrine exposure. This suggests that CNP modulates heart rate indirectly via sympathetic innervation rather than a direct effect on the SA node. This is confirmed ex vivo by experiments showing that CNP decreases neuronal calcium transients and impairs the release of norepinephrine, and in vivo by studies showing that the centrally acting sympatholytic agent clonidine attenuates the hypertension and tachycardia seen in NPR-B transgenic mice. These experiments provide a clear physiological role for CNP in mitigating sympathetic neurotransmission, with direct implications for pathophysiological states such as HF (Figure 1).
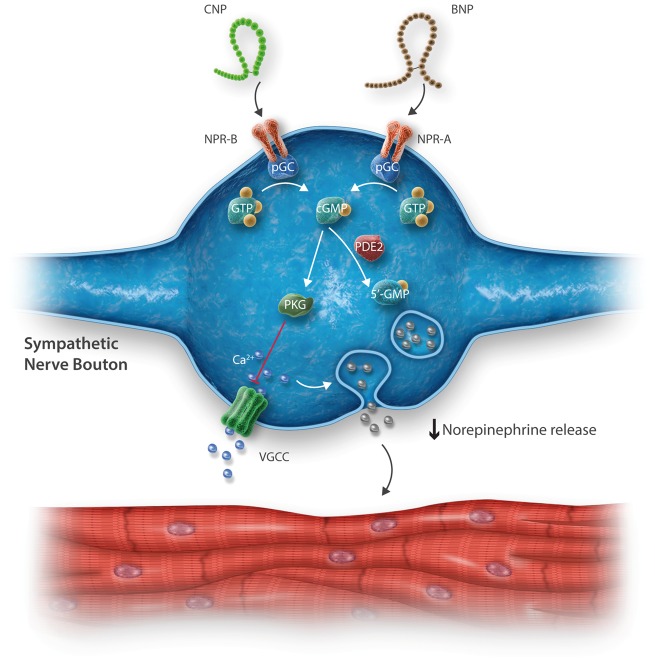
Figure 1.
Simplified schematic illustrating the intracellular signalling cascade potentially utilized by B-type and C-type natriuretic peptide (BNP and CNP, respectively) and their corresponding natriuretic peptide receptors (NPR) A and B. Binding of NP-NPR binding activates particulate guanylyl cyclase (pGC), producing cylic guanosine monophosphate (cGMP) from guanosine triphosphate (GTP) as a result. cGMP activates protein kinase G (PKG, also known as cGMP-activated protein kinase). PKG inhibits calcium influx via voltage gated calcium channels (VGCC), which are activated upon neuronal depolarization. When phosphodiesterase 2 levels are elevated (as seen in chronic sympathoexcitation e.g., spontaneously hypertensive rat), the ability of BNP, and possibly CNP to reduce neurotransmitter release is diminished due to PDE2-mediated degradation of cGMP into 5’GMP.
The rise in CNP levels in HF could act as a ‘check’ to balance sympathoexcitation and intra- and extra-cardiac neural remodelling driven by a number of mechanisms including spinal and renal afferent activation5,6. For instance, CNP exerts protective effects against angiotensin-mediated adverse cardiac remodelling in mice.7 Further, since it is known that there is a loss of central parasympathetic drive during HF; unchecked sympathoexcitation can be particularly deleterious, resulting in arrhythmias and further pump dysfunction. CNP (along with BNP) may serve in this capacity, acting as a component of the endogenous mechanisms in place to oppose chronic sympathetic activation. At doses in the pathophysiologic range, CNP does not have diuretic or natriuretic effects in normal patients8,9, while other studies also suggest that vasodepressor and natriuretic activity of CNP is weaker than ANP.10 These studies did not assess biomarkers of sympathetic neurotransmission to determine whether CNP-modulated sympathetic signalling, however, they emphasize that the primary action of CNP may be geared towards preventing sympathetic neuron hyperactivity as suggested by this study and others,4,11 and to facilitate vagal neurotransmission as previously shown.12
Although elucidating key mechanisms for CNP-mediated modulation of sympathetic neurotransmission furthers our understanding of NPs as a family, some important considerations are raised regarding what occurs in pathology. Despite the beneficial role that CNP may potentially play in HF, is signalling via the NPR-B receptor also ‘gated’ by PDE2A levels, as was demonstrated for BNP?3 This key question is underscored by the failure of Nesiritide in large clinical trials in the face of promising smaller initial trials,2 which may be explained by this ‘brake’. This may limit any therapeutic potential of CNP, and may even suggest that CNP is merely a marker of the HF state, with no significant activity when PDE2A levels are high. While the actions of CNP predominate via NPR-B, opposing actions may be elicited by binding to NPR-C,13 at least in cardiomyocytes, likely due to degradation of the peptide. Whether and how NPR isoforms are altered in neurons and in myocytes in HF and other pathologic conditions remain unknown.
Thus, while these novel data may potentially identify a new target for sympathetic modulation, characterization of CNP-stimulated NPR-B signalling in pathological states is needed before the potential diagnostic and therapeutic implications can be fully appreciated. The slow pace of developing novel HF therapies available to patients highlights the need for novel pathways that have therapeutic potential, which may extend beyond HF to other cardiovascular diseases. The present study by Buttgereit et al.4 and that of Li et al.3 suggest a back to the ‘A B C’s’ is needed on NP signalling in physiologic and pathophysiologic conditions, and highlights the need for further research in this area.
Conflict of interest: none declared.
Acknowledgments
Funding
This work is supported by HL125730 to O.A.A., and HL068231 and HL093056 to B.A.H, from the National Institutes of Health.
References
- 1.Potter LR, Yoder AR, Flora DR, Antos LK, Dickey DM. Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handb Exp Pharmacol 2009;191:341–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fajardo J, Heywood JT, Patterson JH, Adams K, Chow SL. Natriuretic peptides for the treatment of acute heart failure: a focus on nesiritide in recent clinical trials. Expert Rev Cardiovasc Ther 2015;13:743–751. [DOI] [PubMed] [Google Scholar]
- 3.Li D, Lu CJ, Hao G, Wright H, Woodward L, Liu K, Vergari E, Surdo NC, Herring N, Zaccolo M, Paterson DJ. Efficacy of B-type natriuretic peptide is coupled to phosphodiesterase 2A in cardiac sympathetic neurons. Hypertension 2015;66:190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buttgereit J, Shanks J, Li D, Hao G, Athwal A, Langenickel TH, Wright H, da Costa Goncalves AC, Monti J, Plehm R, Popova E, Qadri F, Lapidus I, Ryan B, Ozcelik C, Paterson DJ, Bader M, Herring N. C-type natriuretic peptide and NPR-B signalling inhibits cardiac sympathetic neurotransmission and autonomic function. Cardiovasc Res 2016;112:637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Habecker BA, Anderson ME, Birren SJ, Fukuda K, Herring N, Hoover DB, Kanazawa H, Paterson DJ, Ripplinger CM. Molecular and cellular neurocardiology: development, and cellular and molecular adaptations to heart disease. J Physiol 2016;594:3853–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shivkumar K, Ajijola OA, Anand I, Armour JA, Chen PS, Esler M, De Ferrari GM, Fishbein MC, Goldberger JJ, Harper RM, Joyner MJ, Khalsa SS, Kumar R, Lane R, Mahajan A, Po S, Schwartz PJ, Somers VK, Valderrabano M, Vaseghi M, Zipes DP. Clinical neurocardiology defining the value of neuroscience-based cardiovascular therapeutics. J Physiol 2016;594:3911–3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Izumiya Y, Araki S, Usuku H, Rokutanda T, Hanatani S, Ogawa H. Chronic C-type natriuretic peptide infusion attenuates angiotensin ii-induced myocardial superoxide production and cardiac remodeling. Int J Vasc Med 2012;2012:246058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barletta G, Lazzeri C, Vecchiarino S, Del Bene R, Messeri G, Dello Sbarba A, Mannelli M, La Villa G. Low-dose C-type natriuretic peptide does not affect cardiac and renal function in humans. Hypertension 1998;31:802–808. [DOI] [PubMed] [Google Scholar]
- 9.Hunt PJ, Richards AM, Espiner EA, Nicholls MG, Yandle TG. Bioactivity and metabolism of C-type natriuretic peptide in normal man. J Clin Endocrinol Metab 1994;78:1428–1435. [DOI] [PubMed] [Google Scholar]
- 10.Pham I, Sediame S, Maistre G, Roudot-Thoraval F, Chabrier PE, Carayon A, Adnot S. Renal and vascular effects of C-type and atrial natriuretic peptides in humans. Am J Physiol 1997;273:R1457–1464. [DOI] [PubMed] [Google Scholar]
- 11.Mutafova-Yambolieva VN, Westfall DP. Modulatory effects of type-C natriuretic peptide on sympathetic cotransmission in the rat isolated tail artery. Clin Exp Pharmacol Physiol 1998;25:1013–1017. [DOI] [PubMed] [Google Scholar]
- 12.Herring N, Zaman JA, Paterson DJ. Natriuretic peptides like NO facilitate cardiac vagal neurotransmission and bradycardia via a cGMP pathway. Am J Physiol Heart Circ Physiol 2001;281:H2318–2327. [DOI] [PubMed] [Google Scholar]
- 13.Azer J, Hua R, Vella K, Rose RA. Natriuretic peptides regulate heart rate and sinoatrial node function by activating multiple natriuretic peptide receptors. J Mol Cell Cardiol 2012;53:715–724. [DOI] [PubMed] [Google Scholar]