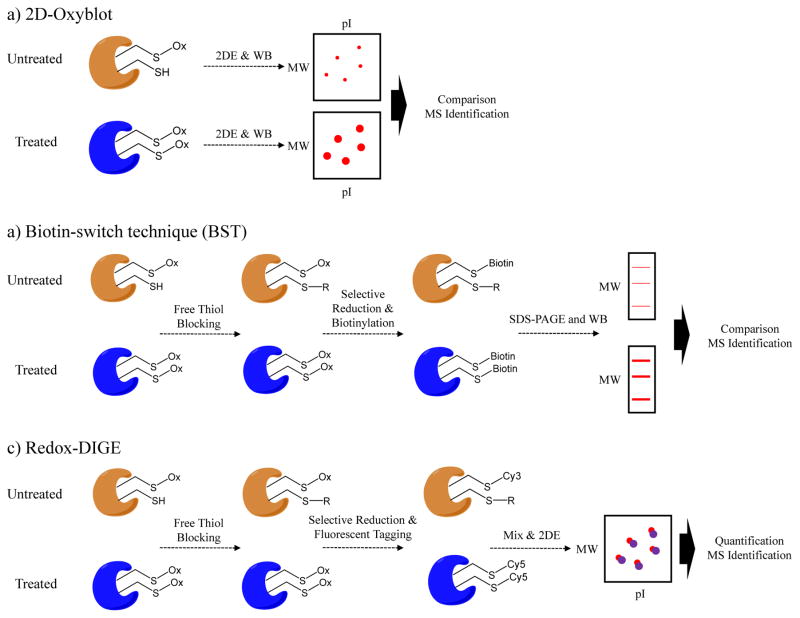
Figure 3.
Gel-based approaches for quantification of cysteine reversible modifications. a) In 2D-Oxyblot method first samples are separated by 2D SDS-PAGE. Protein bands are transferred to a membrane and the blots are probed using antibodies that recognize the particular cysteine PTM, such as S-nitrosylation or S-glutathionylation. Differentially-expressed protein spots are excised and identified by MS. b) Biotin-switch technique (BST) employs differential thiol blocking followed by selective reduction of the cysteine modification. The nascent thiols are labeled with biotin-HPDP. The oxidized proteins are then enriched by avidin affinity medium and analyzed by SDS-PAGE and Western blot. c) In Redox-DIGE method, different fluorescent tags are used to label samples after differential thiol blocking and selective reduction of cysteine modifications. Two samples are combined and analyzed on a single gel. The protein spots with differential fluorescent signals are excised and analyzed by MS.