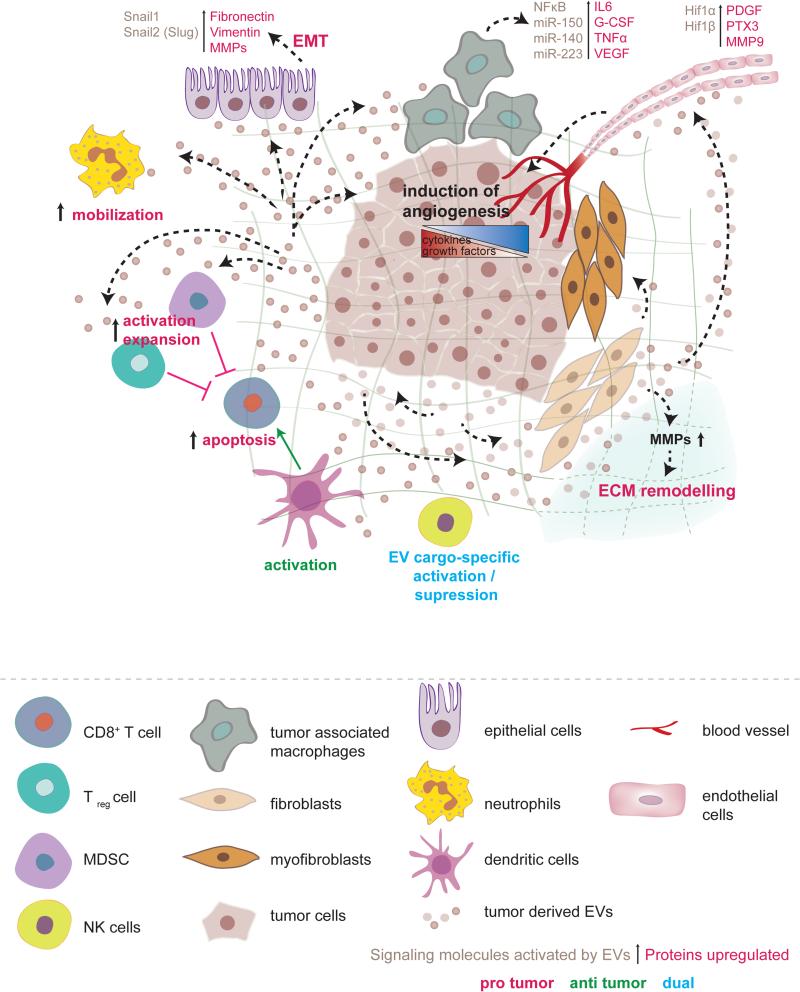
Figure 1. Role of tumor-derived EVs on the primary tumor microenvironment.
Tumor EVs cause fibroblasts to differentiate into myofibroblasts, which release MMPs and lead to extracellular matrix remodeling. The breakdown of ECM leads to the release of growth factors embedded in the ECM and promotes invasion through parenchymal cells. Tumor EVs activate tumor-associated macrophages to secrete G-CSF, VEGF, IL-6, and TNFα, which together promote angiogenesis and create an inflammatory niche. Tumor EVs affect immune system homeostasis mostly by triggering immunosuppressive changes that protect the tumor. Tumor EVs activate and expand Tregs and MDSCs, which inhibit CD8+ T-cell mediated targeting of the tumor. Furthermore, tumor EVs have been shown to express FasL and TRAIL on their membrane and directly induce apoptosis of CD8+ T-cells. Tumor EVs increase neutrophil mobilization and to be associated with increased tumor progression. Natural killer cells, which play an important role in antitumor immunity, can be either activated or suppressed by tumor EVs, depending on the tumor model studied and the EV cargo. Dendritic cells can be activated by the tumor-derived antigens delivered via tumor EVs and enforce a CD8+-mediated anti-tumor response. As the tumor grows, it develops metabolic demands that outgrow its blood supply and thus becomes increasingly hypoxic. In response to low oxygen concentrations, the tumor secrets angiogenic factors and EVs that promote blood vessel recruitment. EVs derived from the primary tumor can stimulate epithelial cells to release factors involved in EMT that trigger the loss of tumor cell adhesion (Fibronectin and Vimentin), ECM remodeling, and angiogenesis (MMPs), which together promote the release of tumor cells into the circulation and their spread to distant sites.