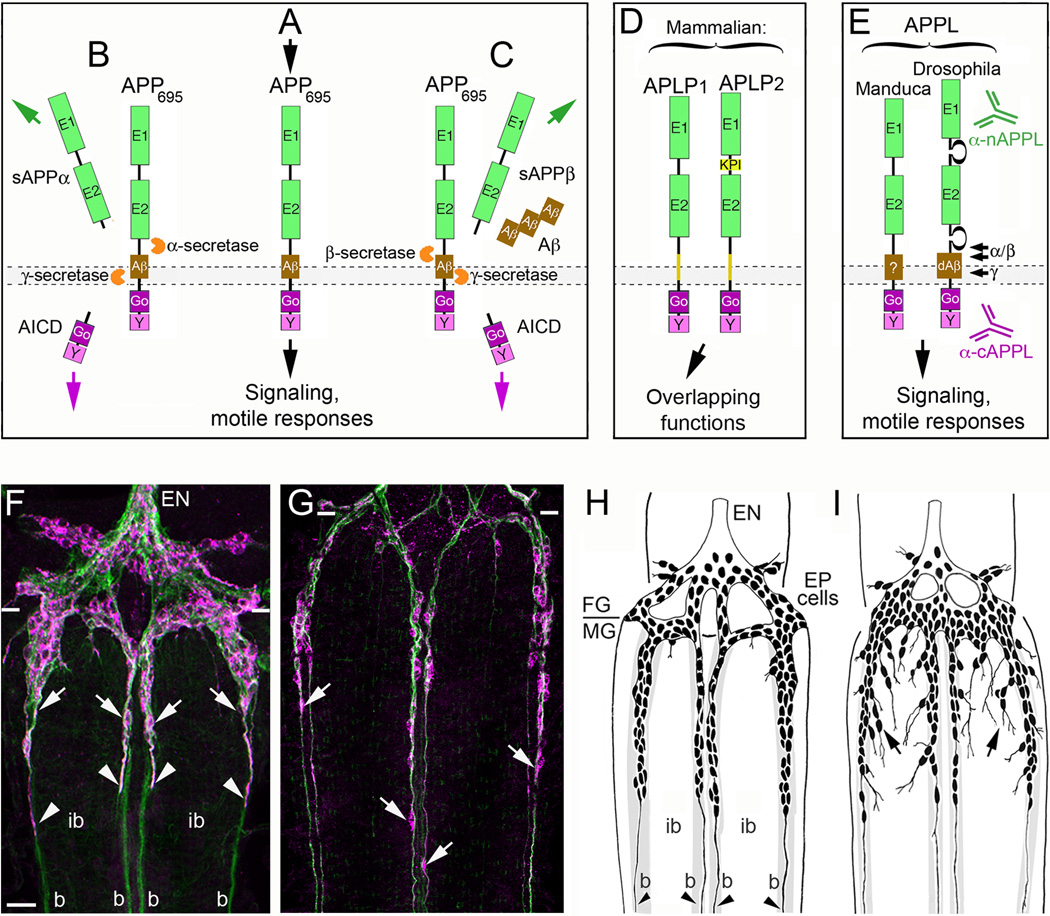
Figure 2. The insect ortholog of Amyloid Precursor Protein regulates neuronal migration in the developing ENS.
(A–E), the structure and processing of APP family proteins is similar in insects and mammals. (A), human APP695 (containing 695 amino acids) has the topology of a type-1 transmembrane glycoprotein, consisting of two extracellular protein interaction domains (E1 and E2); a transmembrane domain that contains the Aβ cleavage fragment; and a short cytoplasmic tail that contains highly conserved binding domains for the heterotrimeric G protein Goα (Go) and a tyrosine-based sorting motif (Y). A wide variety of potential binding proteins and ligands have been identified that can interact with the E1–E2 extracellular domains, while numerous intracellular adapter and signaling proteins besides Goα (are capable of interacting with the cytoplasmic domains. Studies in a variety of systems have shown that APP695 is capable of functioning as a transmembrane receptor, whereby activation with candidate ligands can induce signaling responses that modulate neuronal motility. (B), In the non-amyloidogenic pathway of APP processing, APP is first cleaved by α-secretases at a juxtamembrane site within the Aβ domain, which releases a soluble/secreted ectodomain fragment (sAPPα) and a short transmembrane C-terminal fragment (CTF; not shown). CTF fragments are then rapidly cleaved by the γ-secretase complex (containing presenilins) to produce a cytoplasmic APP intracellular domain (AICD) and a small “p3” peptide of no apparent significance (not shown). (C), In the amyloidogenic pathway, APP is first cleaved by β-secretase (BACE) to generate a slightly shorter sAPPβ ectodomain fragment and a slightly longer CTF fragment containing the Aβ peptide (not shown). This intermediate fragment is then rapidly cleaved by the γ-secretase complex to generate an identical AICD fragment and β-amyloid peptide fragments (Aβ40–42) of varying lengths that accumulate in the brain with aging. Secreted sAPP ectodomain fragments have been ascribed a variety of functions (both beneficial and harmful to neurons), including activation of APP signaling (via interactions with the transmembrane holoprotein); AICD fragments have been shown to induce changes in gene transcription (analogous to the Notch intracellular domain; NICD), although the biological significance of these activities remains under debate. (D), In addition to APP, vertebrates also express to closely related orthologs: APP-Like Protein 1 & 2 (APLP1 and APLP2). Both family members contain extracellular and intracellular protein interaction domains that are closely similar to these domains in APP and have been shown to have partially overlapping functions within the nervous system. (E), Insects only express a single APP family protein, APPL (APP-Like). They also contain similar extracellular and intracellular domains that share considerable sequence conservation with human APP695, including 100% conservation within the Go domain (required for direct interactions with Goα; [79]). Drosophila APPL has also been shown to contain an Aβ-like fragment (dAβ) that is generated by sequential cleavage of APPL by endogenous β- and γ-secretases [70]). Antibodies specific for the n-terminal (α-nAPPL) and c-terminal (α-cAPPL) regions of APPL have been generated that can distinguish the distribution of the holoprotein from its cleavage fragments. (F–G), The embryonic ENS of Manduca at different developmental stages, labeled with anti-TM-Fas II (green) and anti-cAPPL (magenta). (F), At 58 hpf, TM-Fas II is expressed by both the EP cells and their muscle band pathways on the midgut (b). The migratory EP cells also strongly express APPL (arrows) as they travel onto the bands while largely avoiding the adjacent interband regions (ib). Previous studies have shown that transmembrane APPL traffics into their leading processes (arrowheads), where it interacts with Goα [79]). (G), By 65 hpf, the EP cells have transitioned from migration to axon outgrowth, but they continue to robustly express APPL in their cell bodies (arrows) and advancing growth cones (out of the field of view). Paired white hatchmarks indicate the foregut-midgut boundary; scale bar = 30 µm. (H–I), examples of the ENS in embryos that were opened to expose the developing ENS prior to the onset of EP cell migration (~50 hpf) and allowed to develop for an additional 18 hr (through the periods of migration and outgrowth). At the completion of the culture period, embryos were fixed and immunostained with anti-Fas II to reveal the extent of migration and outgrowth, and analyzed by camera lucida methods. (H), Embryo that was treated with control antisense morpholino constructs with no known gene targets in insects; EP cell migration and axon outgrowth (arrowheads) was largely confined to the normal band pathways. (I), Embryo that was treated with antisense morpholino constructs specific for Manduca APPL mRNA; although EP cells that maintained strong contact with their bands migrated and extended axons normally along these pathways, a substantial number of neurons migrated and extended processes inappropriately onto the interband regions (black arrows). A similar pattern of ectopic migration was caused by inhibiting the heterotrimeric G protein Goα or by blocking Goα-dependent Ca2+ influx [79,81].