Abstract
Background
Limited studies have evaluated the role of diffusion weighted imaging (DWI) and apparent diffusion coefficient (ADC) for histologically grading the hepatocellular carcinoma (HCC).
Objective
To compare the efficacy of DWI with dynamic contrast enhanced magnetic resonance (DCEMR) in detection of HCC in cirrhosis, and to evaluate whether DWI can be used instead of DCEMR.
Methods
20 patients of either sex with cirrhosis and suspected of having HCC on screening USG were included in this prospective study approved by the Institutional Ethics Committee. All patients underwent DCEMR of the abdomen on 3T scanner and fine needle aspiration of the lesions. MR protocol included T1WI, T2WI, DWI, and dynamic CEMR. The results of diffusion weighted imaging were compared with DCEMR to find the efficacy of DWI vis-à-vis CEMR.
Results
DWI had a sensitivity and specificity of 100%, for diagnosis of lesions in cases having single lesion on CEMR, and sensitivity of 75% and specificity of 100% for diagnosis of lesions in cases having multiple lesions. There was a decreasing trend of ADC values with increasing grade of the tumor; however, the decreasing trend was not statistically significant. A cut-off ADC value of 0.8705 resulted in a sensitivity of 75% and specificity of 50% for differentiating between well-differentiated and other grades of HCC.
Conclusion
DWI can be used as an alternative for the detection and characterization of HCC, especially in patients with impaired renal function or contrast allergies precluding the use of contrast. In addition, DWI with ADC measurement may be helpful for non-invasive and preoperative prediction of the degree of differentiation of HCC.
Abbreviations: ADC, apparent diffusion coefficient; DCEMR, dynamic contrast enhanced magnetic resonance; DWI, diffusion weighted imaging; HASTE, half-Fourier acquisition single-shot turbo spin echo; HCC, hepatocellular carcinoma; TSE, turbo spin echo; VIBE, volume interpolated breath hold examination
Keywords: HCC, DWI, ADC, grading, DCEMR
Hepatocellular carcinoma (HCC) is the sixth most commonly encountered cancer worldwide and third most common cause for the cancer-related death globally. It is the most common primary hepatic malignancy with around 80% cases developing predominantly in patients who have underlying cirrhotic background.1, 2, 3, 4 Arterial phase enhancement is considered as the essential characteristic of HCC and dynamic contrast enhanced magnetic resonance (DCEMR) imaging is considered as the gold standard for the diagnosis of HCC non-invasively.5 Patients having impaired renal function or history of contrast allergies cannot be administered gadolinium contrast agent for DCEMR, and hence an alternative imaging technique is required for the detection and characterization of HCC.
Diffusion weighted MR imaging (DW-MRI) has emerged as a non-invasive sequence which works on microscopic motion of water in tissue. It is being increasingly used in liver diseases due to availability of echo-planar imaging and parallel imaging techniques which has considerably improved the quality of images with reduction of artifacts related to breathing, cardiac motion, and bowel peristalsis.6 Even though previous studies have reported DWI to have improved sensitivity when combined with CEMR in diagnosing HCC, to the best of our knowledge, there is no previous prospective study directly comparing the efficacy of diffusion weighted imaging (DWI) against DCEMR in the diagnosis of HCC on a 3T MRI scanner. Among the several forecasters of recurrence of HCC after surgical resection, histological grading is the most important foretelling factor for early tumoral recurrence.7, 8 Hence, preoperative prediction of grade of tumor may be helpful for clinicians for further management. It has been hypothesized that highly cellular tumors are associated with more restriction of motion of water molecules, and hence apparent diffusion coefficient (ADC) values are considerably lower for malignant lesions compared to lesions of benign nature and also for high grade tumors than for low grade tumors.6, 9
Limited studies have evaluated the role of DWI and ADC for histologically grading the HCC. However, they have revealed contradicting results for correlation between grade of HCC and ADC values. Hence, the results at present are equivocal for correlation of ADC values with tumor grading. Thus, in the present study, DWI efficacy was compared with DCEMR in detection of HCC in cirrhotics, taking DCEMR as gold standard. We also evaluated whether DWI can be used instead of DCEMR. This can avoid the use of intravenous contrast which will be more beneficial to patients with hepatic and renal failure. The correlation between calculated ADC values and grades of tumor was also looked for, and cut-off ADC value for various grades of HCC was looked for.
Materials and Methods
The prospective study was approved by the Institutional Ethics Committee of Post Graduate Institute of Medical Education and Research, Chandigarh, India. Informed written consent was obtained from all patients. The fine needle aspirate of the focal hepatic lesions was also approved, as we needed to grade the lesion as a part of the study protocol.
Study Population
A total of 20 patients of either sex who presented to out-patient department (OPD) of hepatology clinic of our institute over a period of 18 months, with chronic liver disease and suspected of having HCC on screening USG were included. None of them had undergone previous locoregional therapy or surgery for the liver lesion.
These twenty patients (19 men and one female) were in the age range of 40–80 years with a mean age of 59.05 years. The underlying cause of cirrhosis was HBV in 9 patients, HCV in 6 patients, alcoholic cirrhosis in 4 patients, and non-alcoholic steatohepatitis (NASH) in one patient. The mean AFP level in the study group was 80.13 ng/ml with 14 patients having AFP levels less than 100 ng/ml.
Imaging Protocol
MR abdomen was done on 3T (SIEMENS VERIO) imaging system using a body coil. The protocol included T1 weighted axial imaging, T2 weighted axial imaging including both non fat suppressed and fat suppressed sequence, axial DWI and conventional axial DCEMR. The T1-weighted gradient echo sequence had following parameters: TR—120 ms, TE—2.46 ms, flip angle—65°, slice thickness—6 mm, interslice gap—1 mm, matrix—320 × 320, and field of view—38 × 38 cm. The T2-weighted half-Fourier acquisition single-shot turbo spin echo (HASTE) sequence had following parameters: TR—2000 ms, TE—96 ms, flip angle −150°, slice thickness—6 mm, interslice gap—1 mm, matrix—320 × 320, and field of view—38 cm × 38 cm. Fat suppressed T2-weighted turbo spin echo (TSE) sequence was obtained with simultaneous use of respiratory gating with following parameters: TR/TE—6600–8600 ms/84 ms, slice thickness—6 mm, interslice gap—1 mm, matrix—320 × 320, and field of view—38 cm × 38 cm. The DWI (single shot echo planar imaging with simultaneous use of respiratory triggering) was obtained with following parameters: TR/TE—6600–8400 ms/92 ms, slice thickness—6 mm, interslice gap—1 mm, field of view—38 cm × 38 cm, and matrix—320 × 320. The acquisition was done with four b values of 0, 100, 500, and 1000 s/mm2 with diffusion encoding gradient in all three orthogonal directions. DWI sequence was of around 4–5 min. The trace images were obtained separately for all the four b values. Imaging software on the imager console automatically generated a pixel-based ADC map. The DCEMR images were obtained using 3D gradient echo FLASH (volume interpolated breath hold examination, VIBE) sequence using following parameters: TR/TE—6600–8400 ms/92 ms, slice thickness—6 mm, interslice gap—1 mm, field of view—38 cm × 38 cm, and matrix—320 × 320. The images were acquired before injecting contrast and after the time period of 30, 70, and 180 s after injecting contrast corresponding to arterial dominant, portal venous, and delayed phase imaging. Gadobenate dimeglumine (multihance) contrast was injected (0.1 mmol/kg of contrast material) through antecubital vein using a power injector at a rate of 2–3 ml/s. Subtraction images were generated automatically on the console by the imager software for every set of post-contrast images for assessing enhancement in nodules that were of high signal intensity on T1-weighted images before injection of gadolinium.
Image Interpretation
The conventional sequences including T1 WI and T2 WI were interpreted for presence of lesion, number of lesions, and for signal characterization of lesion in both the sequences. The DWI and DCEMR images were interpreted separately by two different radiologists. Both of them had more than 10 years of experience in abdominal imaging. The lesions which showed enhancement in hepatic arterial phase with or without washout in delayed images were considered positive in DCEMR for HCC. The lesions which appeared bright on DWI and dark on ADC maps were considered positive in DWI for HCC. On ADC maps, ADC values of the lesion were measured by placing region of interest (ROI) manually within the lesion. At least five ROIs of uniform size of 44 pixels were taken within the lesion and mean ADC value was calculated. While measuring the ADC values, care was taken to place ROI exactly within the part of lesion corresponding to solid enhancing part of tumor in DCEMR. The care was taken to avoid placing the ROI within the part of tumor corresponding to area of necrosis in DCEMR and T2 WI. In case where there were multiple lesions, the ADC value of the largest lesion was measured.
Cytological Grading of Lesion
The lesions seen on MR were sampled using a 22G LP needle. The cell block specimens were also obtained. In case there were multiple lesions, sampling of the largest lesion was done. All the lesions were interpreted by a single pathologist having more than 10 years of experience and was blinded to the findings of MRI. The lesions were categorized cytologically as well, moderate, and poorly differentiated based on Edmondson grading system. The staining was done using May-Grünwald Giemsa stain and Hematoxyline-eosin stain.
Statistical Analysis
The statistical analysis was carried out using statistical package for social sciences (SPSS. version 6.0). The degree of agreement between the two modalities DWI and DCEMR was measured using kappa test of agreement. The ADC values of various grades of HCC were tested for normality using Kolmogorov–Smirnov tests. Normally distributed data were expressed as mean ± SD of all the groups. Then, the mean ADC values of various groups were correlated with cytological grading using ANOVA tests. A P value of <0.05 was considered significant in all tests. The cut-off ADC value between various grades was determined by receiver operating characteristic (ROC) curve analysis.
Results
MR Interpretation
Out of 20 patients, single lesion was found in 16 patients, two lesions in one patient, four lesions in another one patient, while multiple lesions were seen in two patients on DCEMR. According to the morphology, the lesions were classified as solitary (75%), multifocal (20%), and infiltrative (5%). The lesions varied in size from 1.3 cm to 17 cm in their maximum dimensions with a mean size of 6 cm. In patients having multiple lesions, the characteristics of the largest lesion were considered for statistical purpose and hence a total of 20 lesions were evaluated. Based on signal intensity, the lesions were interpreted as hypointense (45%), isointense (40%), and hyperintense (15%) on T1WI with presence of T1 hypointense rim seen in 10 (50%) patients. The lesions were classified as either isointense (20%) or hyperintense (80%) on T2WI with presence of T2 hyperintense rim in 2 (10%) patients. None of the lesions were hypointense on T2WI. The lesions were interpreted on CEMR as those showing arterial phase enhancement with washout on delayed images (90%) and those showing arterial phase enhancement without evidence of washout on delayed images (10%). On delayed images, the lesions were classified as either having delayed enhancement of capsule (70%), or as those not having delayed enhancement of capsule (30%).
On DWI, out of 20 patients, 17 patients had single lesion, one patient had four lesions, while two patients had multiple lesions. The lesions in DWI were either homogenously bright (40%) or heterogenously bright (60%). The lesions were similarly either homogenously dark (10%), heterogenously dark (60%), and peripherally dark (30%) on ADC maps.
We tried to calculate the percentage of lesions seen using various criteria such as arterial hypervascularity, washout, and diffusion restriction in diagnosing HCC. For the purpose of calculating the percentages, we excluded the two patients who had multiple lesions diagnosed in both modalities, DCEMR and DWI. Excluding them, we had a total of 22 lesions, out of which a total of 20 lesions were picked among 22 lesions (90.9%) using the criteria of arterial phase enhancement with washout as two patients had single lesion that showed hypervascularity on arterial phase, but no washout on delayed images. The criteria of diffusion restriction again picked up a total of 21 lesions (95.45%), as one patient had one lesion out of two lesions not seen in DWI, but seen on DCEMR. The combined criteria of hypervascularity on arterial phase and washout on delayed images picked up all the lesions as none of the patients had lesions seen on DWI, with not being seen on DCEMR.
Cytological Grading and ADC Values
On pathological examination, the lesions were interpreted as well differentiated in 8 patients (40%), moderately differentiated in 10 patients (50%), and poorly differentiated in 2 patients (10%). The ADC values of the lesions of all grades ranged from 0.5 to 1.1 × l0−3 mm2/s (mean 0.86 ± 0.15 SD). The mean ADC values of poorly differentiated, moderately differentiated, and well-differentiated HCC were 0.69500 ± 0.121622 × l0−3 mm2/s, 0.8664 ± 0.10024 × l0−3 mm2/s, and 0.92410 ± 0.16513 × l0−3 mm2/s respectively. We calculated the ADC value of surrounding liver parenchyma, similarly by placing five ROIs randomly within the surrounding liver parenchyma and calculating the mean ADC value. The mean ADC value of surrounding liver in poorly differentiated, moderately differentiated, and well-differentiated HCC were 1.3210 ± 0.2807 × l0−3 mm2/s, 1.2190 ± 0.1877 × l0−3 mm2/s, and 1.243 ± 0.0962 × l0−3 mm2/s respectively (Figure 1, Figure 2, Figure 3).
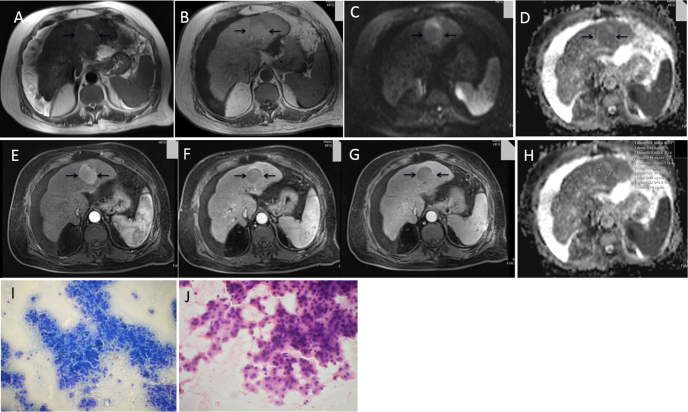
Figure 1.
65-year-old male, a known case of NASH, with history of abdominal pain and loss of appetite and weight (AFP- 16 ng/ml). T2 WI axial (A) and T1 WI axial (B) images show a well-defined SOL, T1 isointense and T2 isointense lesion in left lobe of liver in cirrhotic background. DWI b value 1000 (C) and ADC MAP (D) showing lesion to be homogenously bright on DWI (except for small necrotic area anteriorly) and heterogenously dark on ADC map. DCEMR arterial phase (E), portal venous phase (F) and delayed phase (G) showing lesion to be hypervascular on arterial phase with washout on portal venous phase and enhancement of capsule on delayed image. (H) ADC map with ADC values (mean ADC - 0.941). (I) and (J) microphotograph of FNAC smear (I, May-Grünwald Giemsa stain, original magnification 200× and J, Hematoxyline-eosin stain, original magnification 200×) shows well differentiated HCC.
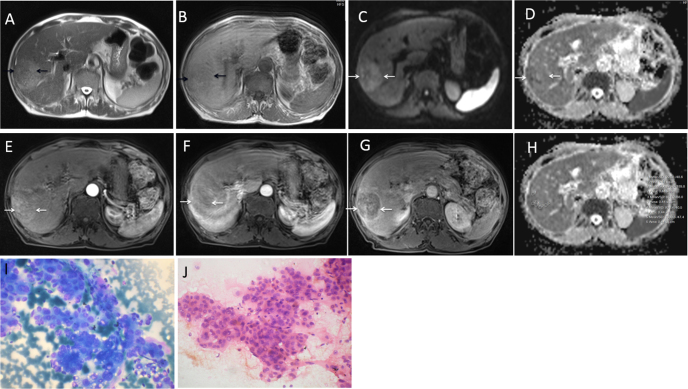
Figure 2.
75-year-old HCV positive male with history of abdominal pain and loss of weight and appetite (AFP- 54.4 ng/ml). T2 WI axial (A) and T1 WI axial (B) axial images show a well-defined SOL, T1 hypointense, and T2 hyperintense lesion in right lobe of liver in cirrhotic background. DWI b value 1000 (C) and ADC map (D) showing lesion to be heterogenously bright on DWI and peripherally dark on ADC map. DCEMR arterial phase (E), portal venous phase (F) and delayed phase (G) showing lesion to be hypervascular on arterial phase with washout on portal venous phase and enhancement of capsule on delayed image. (H) ADC map with ADC values (mean ADC- 0.908). (I) and (J) microphotograph of FNAC smear (I, May-Grünwald Giemsa stain, original magnification 400× and J, hematoxyline-eosin stain, original magnification 400×) shows moderately differentiated HCC.
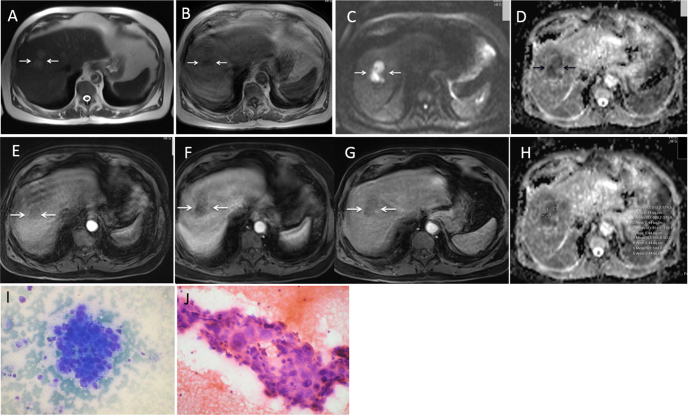
Figure 3.
80-year-old HBV positive male with history of abdominal pain (AFP-110 ng/ml). T1 WI axial (A) and T2 WI axial (B) images show a well-defined SOL, T1 hypointense, and T2 hyperintense lesion in right lobe of liver in cirrhotic background. DWI b value 1000 (C) and ADC MAP (D) showing lesion to be homogenously bright on DWI and homogenously dark on ADC map. DCEMR arterial phase (E), portal venous phase (F), and delayed phase (G) showing lesion to be minimally hypervascular on arterial phase with washout on portal venous phase. (H) ADC map with ADC values (mean ADC- 0.609). (I) and (J) microphotograph of FNAC smear (I, May-Grünwald Giemsa stain, original magnification 200× and J, Hematoxyline-eosin stain original magnification 400×) showing poorly differentiated HCC.
Degree of Agreement Between DWI and DCEMR
DWI and DCEMR had similar findings on 19 patients. There was difference in only one patient in whom DCEMR showed two lesions while DWI picked up only one lesion. The observed kappa value was 0.8984 with a range of 0.6818–1.0. This value corresponds to high degree of agreement between these two modalities.
Correlation of ADC Values With Cytological Grading
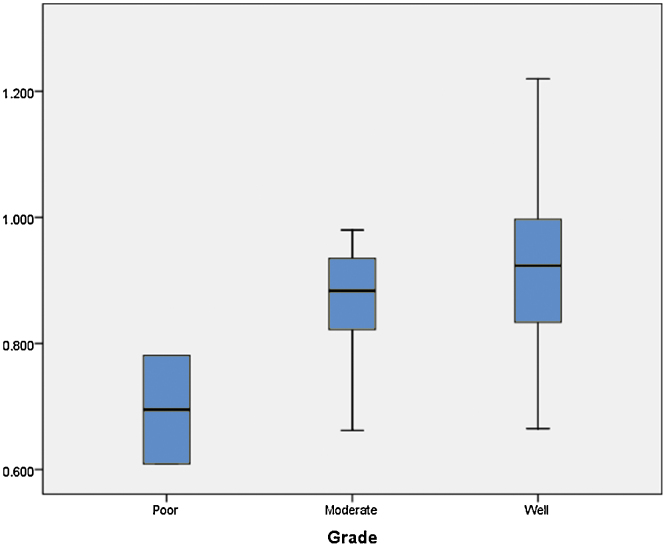
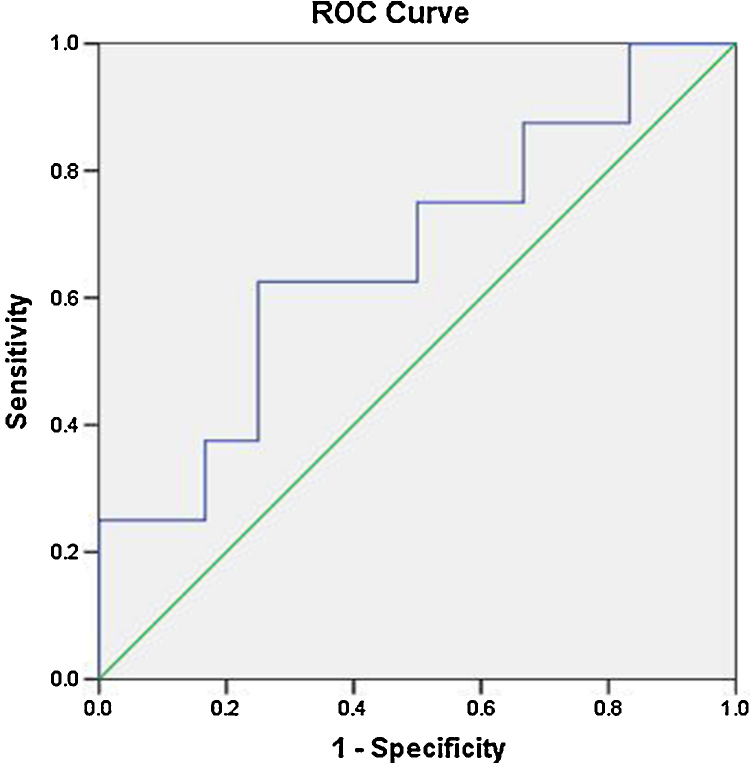
The ADC values of three grades of HCC showed a decreasing trend with increasing grade. The decreasing trend of ADC values is illustrated in Figure 4. However, they did not show any correlation as P value is 0.118 which is not statistically significant. The P values of correlation of ADC values with various grades of HCC are shown in Table 1. When we tried to draw a ROC curve, to define a cut-off value between well-differentiated and other two grades (moderate and poorly differentiated clubbed together) of HCC, we got a cut-off value of 0.87050 × l0−3 mm2/s to differentiate between well and other two grades of HCC with a sensitivity of 75% and specificity of 50%. The ROC curve is illustrated in Figure 5. The ADC values of surrounding liver parenchyma were correlated with cytological grading of tumor using ANOVA test. The mean ADC values of the surrounding liver did not show any correlation with grading of lesion as evidenced by a P value of 0.727.
Figure 4.
Comparison of ADC values of various grades of HCC.
Table 1.
Correlation of ADC Value With Grading.
| Correlation | P value |
|---|---|
| Well, moderate and poor | 0.118 |
| Well and moderate | 1.00 |
| Moderate and poor | 0.336 |
| Well and poor | 0.127 |
Figure 5.
ROC analysis for differentiating between well-differentiated and other grades of HCC.
Discussion
HCC is one of the most common causes of cancer-related death. Patients who are diagnosed at an early stage without metastasis are eligible for curative therapies and hence have a good prognosis in the range of 50–70% 5-year survival rate.10 However, the prognosis is poor when HCC is diagnosed at an advanced stage. Hence, diagnosing HCC at an early stage is important. The radiological criteria have been proposed for diagnosing HCC early without sampling.11 The diagnostic imaging criteria for HCC include arterial phase enhancement with washout in the portal venous and/or delayed phases on computed tomography or magnetic resonance imaging.12, 13 However, gadolinium contrast cannot be used in patients with chronic renal failure due to risk of nephrogenic systemic fibrosis and in those with history of allergy to gadolinium. This creates a need for imaging sequences without the use of gadolinium which can be used for diagnosing HCC in patients with contraindication for gadolinium. In DCEMR, hepatic lesions, which show arterial phase enhancement without venous washout such as dysplastic nodules, and arterioportal shunts are often encountered which often pose a diagnostic difficulty in discriminating HCC from these pseudo-enhancing lesions.14 Also, a small percentage of HCC are hypovascular which do not show enhancement in arterial phase images. When these standard criteria are used, a sensitivity of 59.6% was calculated for diagnosing of HCC by DCEMR in the study by Piana et al.,15 whereas it was slightly higher in the study by Forner et al.16 reaching 61.7%. This low sensitivity is mainly due to arterial phase enhancing lesions not showing washout in the venous or delayed phase images and to a less extent due to hypovascular HCCs, which do not enhance in arterial phase. Since arterial phase enhancement is also seen in many lesions other than HCCs, the washout on delayed images is a criterion to increase specificity which in turn decreases the sensitivity of the criterion.13 Hence, to increase the sensitivity, there arises a need for defining additional imaging criteria.
DWI shows microscopic motion of water in tissue which involves exploitation of movement of water molecules in the blood vessels, and extracellular and intracellular spaces.17 To the best of our knowledge, even though few studies have compared the combined efficacy of DWI and DCEMR to that of DCEMR alone, this is the first prospective study in which efficacy of DWI alone is directly compared with DCEMR. DWI was outperformed by CEMR for detection of HCC in the retrospective study by Park et al.18 However, it represented a reasonable alternative to CEMR for detection of HCC with a size above 2 cm. The addition of DWI to CEMR slightly increased the detection rate in their study. In the present study, DWI had a high degree of agreement (kappa co-efficient-0.8984) with DCEMR, identifying almost all lesions except for single lesion in one patient. In our study, 95.45% of HCCs were hyperintense on DWI. This percentage is slightly higher than in previous studies. In the study by Piana et al.,15 82% or 72% of HCC were hyperintense depending on the reader, where around 91.2% of hypervascular HCC were hyperintense in the study by Nasu et al.19
Previous reports have also mentioned that it may be difficult to differentiate HCC from other solid hepatic lesions like focal nodular hyperplasia (FNH), adenomas, and metastasis by DWI. As these solid lesions also have increased cellularity, they can show ADC values that overlap with ADC values of HCC.20, 21 However, the objective of the present study was to assess the role of DWI in cirrhotic patients only. According to the recent International Working Party's Terminology of Nodular Hepatocellular Lesions, FNH and adenoma should not be diagnosed within a cirrhotic liver.22 Furthermore, metastases to a liver with a cirrhotic background are very unlikely due to reduction of portal blood flow. With these results in background, DWI can be confidently applied as a single sequence for diagnosing HCC in patients with liver cirrhosis and in whom there exists a contraindication to gadolinium. In patients with non-cirrhotic liver, when a liver lesion is detected on conventional sequences but is not very characteristic of HCC, the imaging findings on DWI may add confidence for detection and characterization of HCC.
In the second part of the present study, attempt was made to find out whether quantitative analysis of ADC and DWI before treatment can help in assessment of cytological grading. Taouli et al. first showed that ADC values can be used to differentiate between benign and malignant lesions in liver.20, 21 After this, few studies23, 24 were done to differentiate between benign and malignant hepatic lesions and also to differentiate between various grades of HCC. Nasu et al.,19 Piana et al.,15 and Saito et al.25 showed that there exists no relationship between ADC values and histopathological grades of HCC, whereas Heo et al.26 and Nishie et al.27 said that the ADC values of HCC had an inverse correlation with the degree of differentiation. In the present study, even though there was a decreasing trend of ADC values with increasing grade of tumor, however the decreasing trend was not statistically significant (P value of 0.118).
To the best of our knowledge, this is the first study done on a 3T MR scanner, whereas previous published studies have been limited to 1.5T. With increase in field strength, the increased signal to noise ratio (SNR) can be used to improve spatial resolution or achieve a higher diffusion weighting, which can thereby increase the accuracy of diffusion studies. Most of the previous studies used only two b values, whereas in our study, we used a maximum of four b values. Multiple b values help in calculating a precise ADC value with decreased regional ADC variations and decreased perfusion contamination. Nasu et al.19 and Piana et al.15 in their studies used a maximum b value of 500 mm2/s, whereas in our study, we used a maximum b value of 1000 mm2/s. A high b value of 1000 s/mm2 in addition provides information about the water molecules which have a slow diffusion and thereby reflecting relatively minor effects like intracellular microenvironment. Nasu et al.19 and Piana et al.15 in their studies had the fallacy of not taking into account the necrotic areas while measuring ADC of the lesion, whereas in our study, we tried to avoid the necrotic areas in the measurement of ADC value by placing ROI in ADC maps corresponding to solid enhancing part of tumor in DCEMR.
Generally, cytopathological grade of a neoplasm is determined by both cellular and nuclear atypia.28 In the present study, classification the HCCs was done based on Edmondson-Steiner grading system,29 which primarily depends on nuclear atypia that affects the intracellular environment rather than the intercellular environment. Nuclear atypia, which is represented mainly by the nuclear-cytoplasmic ratio, is not fully represented on the DWI sequence. DWI primarily describes the Brownian motion of the extracellular water molecules with little description of movement of intracellular water molecules. This could be one reason as to why ADC values did not show significant correlation with cytopathological grading.
This study had quite a few limitations. Firstly, this was a small study population of 20 patients only. Further studies with increased patient population are recommended. In addition, most of the lesions were more than 2 cm. The efficacy of DWI in detection of small lesions may be evaluated in future studies with relatively large sample population. Secondly, CEMR was taken as gold standard as per AASLD and EASL guidelines and efficacy of DWI was compared with CEMR and not with explanted liver. The lesions that were missed in both the studies could not be determined and were not taken into consideration for calculation of sensitivity and specificity. Thirdly, only FNAC was taken from the lesion and ADC values were correlated with cytopathological grading. Most of the published studies have used histopathologic specimens for correlation. Though we used cell blocks for grading, histopathological grading is the gold standard when compared to cytopathological grading. Fourthly, the ADC value measurement in MR imaging may not exactly co-register with the cytological specimen meaning that area from which the ADC values were measured may not be exactly sampled during cytological examination. The ADC values were measured by drawing five different ROIs in the same slice of tumor, but not including the cystic or necrotic components. This sampling bias could have contributed to the disparity between the ADC values and the cytopathologic analysis since drawing multiple ROIs in one slice may not fully represent the tumoral cytopathological heterogeneity. Finally, most of the patients turned out to have either well or moderately differentiated HCC with only two of the patients belonging to poorly differentiated HCC and hence we were not able to define a cut-off between poorly differentiated and other grades of HCC.
To conclude, DWI is seen to have excellent degree of agreement with DCEMR for detection of HCC. In patients with impaired renal function or contrast allergies precluding use of dynamic gadolinium enhanced imaging, DWI can be used as an alternative for the detection and characterization of HCC. Although correct prediction of exact histopathological grade of HCC is not possible because of the large overlap among the ADC values, DWI with ADC measurement may be helpful for non-invasive and preoperative prediction of the degree of differentiation of HCC. However, further studies with increasing number of patients are recommended.
Conflicts of Interest
The authors have none to declare.
References
- 1.Bruix J., Llovet J.M. Major achievements in hepatocellular carcinoma. Lancet. 2009;373:614–616. doi: 10.1016/S0140-6736(09)60381-0. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag H.B. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol. 2002;35:72–78. doi: 10.1097/00004836-200211002-00002. [DOI] [PubMed] [Google Scholar]
- 3.Kalra N., Kang M., Bhatia A. Role of radiofrequency ablation in unresectable hepatocellular carcinoma: an Indian experience. Indian J Radiol Imaging. 2013;23:139–144. doi: 10.4103/0971-3026.116569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalra N., Gupta P., Chawla Y., Khandelwal N. Locoregional treatment for hepatocellular carcinoma: the best is yet to come. World J Radiol. 2015;28:306–318. doi: 10.4329/wjr.v7.i10.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelekis N.L., Semelka R.C., Worawattanakul S. Hepatocellular carcinoma in North America: a multiinstitutional study of appearance on T1-weighted, T2-weighted, and serial gadolinium-enhanced gradient echo images. AJR Am J Roentgenol. 1998;170:1005–1013. doi: 10.2214/ajr.170.4.9530051. [DOI] [PubMed] [Google Scholar]
- 6.Parikh T., Drew S.J., Lee V.S. Focal liver lesion detection and characterization with diffusion weighted MR imaging: comparison with standard breath-hold T2-weighted imaging. Radiology. 2008;246:812–822. doi: 10.1148/radiol.2463070432. [DOI] [PubMed] [Google Scholar]
- 7.Imamura H., Matsuyama Y., Tanaka E. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–207. doi: 10.1016/s0168-8278(02)00360-4. [DOI] [PubMed] [Google Scholar]
- 8.Regimbeau J.M., Abdalla E.K., Vauyhey J.N. Risk factors for early death due to recurrence after liver resection for hepatocellular carcinoma: result of a multicenter study. J Surg Oncol. 2004;85:36–41. doi: 10.1002/jso.10284. [DOI] [PubMed] [Google Scholar]
- 9.Bruegel M., Holzapfel K., Gaa J. Characterization of focal liver lesions by ADC measurements using a respiratory triggered diffusion-weighted single-shot echo-planar MR imaging technique. Eur Radiol. 2008;18:477–485. doi: 10.1007/s00330-007-0785-9. [DOI] [PubMed] [Google Scholar]
- 10.Bruix J., Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 11.França A.V., Elias Junior J., Lima B.L., Martinelli A.L., Carrilho F.J. Diagnosis, staging and treatment of hepatocellular carcinoma. Braz J Med Biol Res. 2004;37:1689–1705. doi: 10.1590/s0100-879x2004001100015. [DOI] [PubMed] [Google Scholar]
- 12.Shinmura R., Matsui O., Kobayashi S. Cirrhotic nodules: association between MR imaging signal intensity and intranodular blood supply. Radiology. 2005;237:512–519. doi: 10.1148/radiol.2372041389. [DOI] [PubMed] [Google Scholar]
- 13.Marrero J.A., Hussain H.K., Nghiem H.V., Umar R., Fontana R.J., Lok A.S. Improving the prediction of hepatocellular carcinoma in cirrhotic patients with an arterially-enhancing liver mass. Liver Transpl. 2005;11:281–289. doi: 10.1002/lt.20357. [DOI] [PubMed] [Google Scholar]
- 14.Ju Xu P., Hua Yan F., Hua Wang J., Lin J., Ji Y. Added value of breath hold diffusion-weighted MRI in detection of small hepatocellular carcinoma lesions compared with dynamic contrast-enhanced MRI alone. J MRI. 2009;29:341–349. doi: 10.1002/jmri.21650. [DOI] [PubMed] [Google Scholar]
- 15.Piana G., Trinquart L., Meskine N., Barrau V., Van Beers B., Vilgrain V. New imaging criteria with a diffusion-weighted sequence for the diagnosis of hepatocellular carcinoma in chronic liver diseases. J Hepatol. 2011;55:126–132. doi: 10.1016/j.jhep.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 16.Forner A., Vilana R., Ayuso C. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the non-invasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008;47:97–104. doi: 10.1002/hep.21966. [DOI] [PubMed] [Google Scholar]
- 17.Stejskal E.O., Tanner J.E. Spin diffusion measurements: spin-echo in the presence of a time dependent field gradient. J Chem Phys. 1965;42:288–292. [Google Scholar]
- 18.Park M.S., Kim S., Patel J. Hepatocellular carcinoma: detection with diffusion-weighted versus contrast-enhanced magnetic resonance imaging in pre transplant patients. Hepatology. 2012;56:140–148. doi: 10.1002/hep.25681. [DOI] [PubMed] [Google Scholar]
- 19.Nasu K., Kuroki Y., Tsukamoto T., Nakajima H., Mori K., Minami M. Diffusion-weighted imaging of surgically resected hepatocellular carcinoma: imaging characteristics and relationship among signal intensity, apparent diffusion coefficient, and histopathologic grade. AJR Am J Roentgenol. 2009;193:438–444. doi: 10.2214/AJR.08.1424. [DOI] [PubMed] [Google Scholar]
- 20.Touli B., Koh D.M. Diffusion-weighted MR imaging of the liver. Radiology. 2010;254:47–62. doi: 10.1148/radiol.09090021. [DOI] [PubMed] [Google Scholar]
- 21.Taouli B., Vilgrain V., Dumont E., Daire J.L., Fan B., Menu Y. Evaluation of liver diffusion isotropy and characterization of focal hepatic lesions with two single-shot echo-planar MR imaging sequences. Radiology. 2003;226:71–80. doi: 10.1148/radiol.2261011904. [DOI] [PubMed] [Google Scholar]
- 22.International Working Party Terminology of nodular hepatocellular lesions. Hepatology. 1995;22:983–993. doi: 10.1016/0270-9139(95)90324-0. [DOI] [PubMed] [Google Scholar]
- 23.Ki T., Murakami T., Takahashi S., Hon M., Tsuda K., Nakamuna H. Diffusion-weighted single-shot echoplanar MR imaging for liver disease. AJR Am J Roentgenol. 1999;173:393–398. doi: 10.2214/ajr.173.2.10430143. [DOI] [PubMed] [Google Scholar]
- 24.Xu P.J., Yan F.H., Wang J.H., Shan Y., Ji Y., Chen C.Z. Contribution of diffusion weighted magnetic resonance imaging in the characterization of hepatocellular carcinomas and dysplastic nodules in cirrhotic liver. J Comput Assist Tomogr. 2010;34:506–512. doi: 10.1097/RCT.0b013e3181da3671. [DOI] [PubMed] [Google Scholar]
- 25.Saito K., Moriyasu F., Sugimoto K. Histological grade of differentiation of hepatocellular carcinoma: comparison of the efficacy of diffusion-weighted MRI with T2-weighted imaging and angiography-assisted CT. J Med Imaging Radiat Oncol. 2012;56:261–269. doi: 10.1111/j.1754-9485.2012.02374.x. [DOI] [PubMed] [Google Scholar]
- 26.Heo S.H., Jeong Y.Y., Shin S.S. Apparent diffusion coefficient value of diffusion-weighted imaging for hepatocellular carcinoma: correlation with the histologic differentiation and the expression of vascular endothelial growth factor. Korean J Radiol. 2010;11:295–303. doi: 10.3348/kjr.2010.11.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishie A., Tajima T., Ishigami K. Detection of hepatocellular carcinoma (HCC) using super paramagnetic iron oxide (SPIO)-enhanced MRI: added value of diffusion-weighted imaging (DWI) J Magn Reson Imaging. 2010;31:373–382. doi: 10.1002/jmri.22059. [DOI] [PubMed] [Google Scholar]
- 28.Piotr K., Torbenson M., Sheth S., Erozan Ali S. Cytopathological grading of HCC on fine needle aspiration. Cancer. 2004;102:247–252. doi: 10.1002/cncr.20409. [DOI] [PubMed] [Google Scholar]
- 29.Edmondson H.A., Steiner P.E. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]