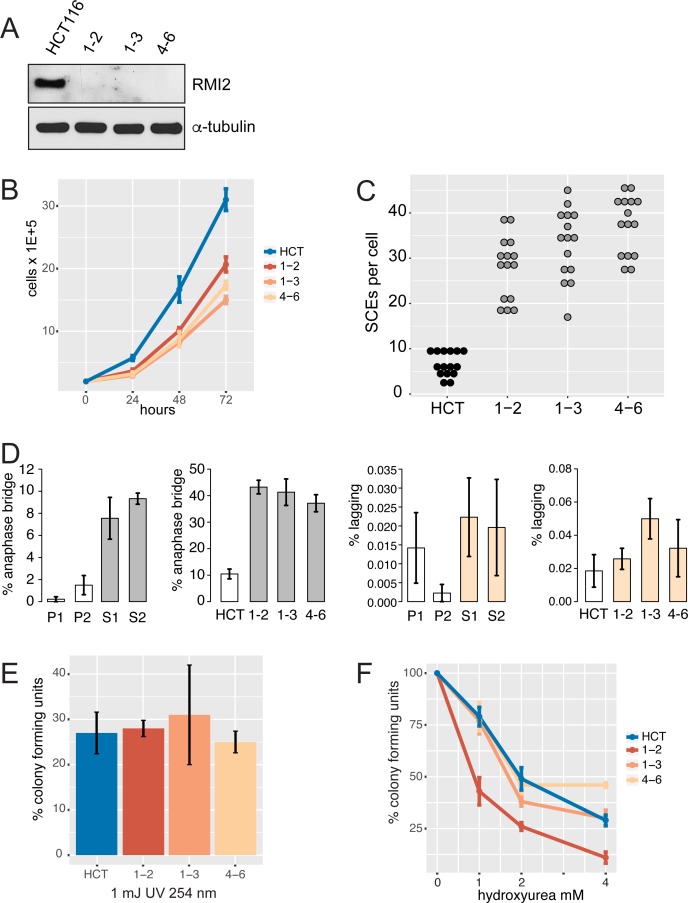
Fig 4. RMI2 cellular defects in knockout cell lines.
(A) Immunoblot of HCT-116 wild type and three independent RMI2 null clones (1–2, 1–3 and 4–6) confirms loss of RMI2 protein in gene knockout cells. Equivalent cell extract (40 μg) was loaded in each lane with anti-α-tubulin used as a loading control. (B) Cell proliferation analysis over three days performed in triplicate for each cell line. (C) Sister chromatid exchange analysis on parental and the three RMI2 null clones. Fifteen metaphase cells were analysed for each cell line. (D) Quantification of anaphase bridges and lagging chromosome from parental heterozygote, P1, P2, and homozygous siblings S1 and S2, and in RMI2 wild type and null HCT-116 cells. For fibroblasts (P1, P2, S1, S2) at least 200 anaphase/telophase cells were scored in total for each line from four independent experiments using matched cell passage number. For HCT-116 cells, at least 200 anaphase/telophase cells were scored for each of wild-type HCT-116, and RMI2 null clones 1–2, 1–3 and 4–6 from three independent experiments using matched cell passage number. Error bars represent standard error of the mean. (E) Colony forming and UV sensitivity assays on HCT-116 and null cell lines. The total number of colonies from three independent experiments are normalised against untreated cells. (F) Colony forming and hydroxyurea sensitivity assays on HCT-116 and null cell lines. Experiments were normalised as in the UV-challenge experiment. Error bars represent standard error of the mean.