Figure 2. Modulating indoxyl sulfate production by controlling the level of BT1492 and its orthologs.
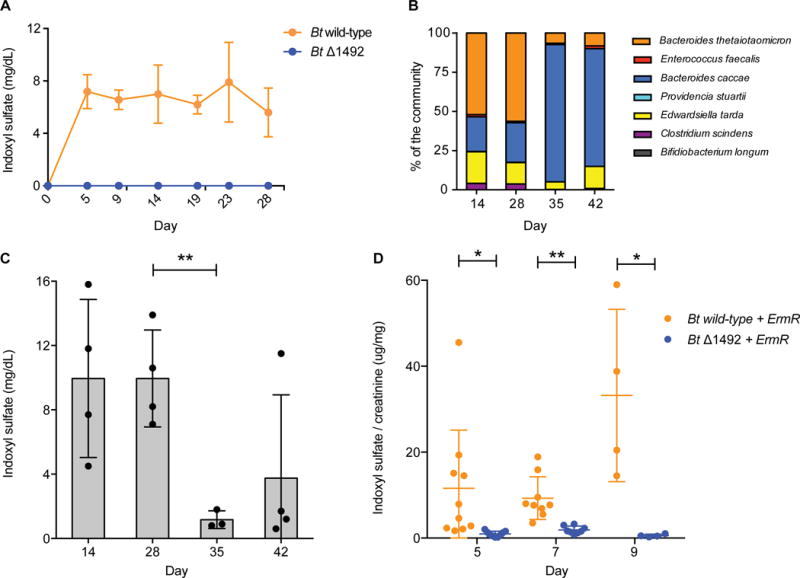
(A) Germ-free mice were mono-colonized with either Bt wild-type or Bt Δ1492 and the level of circulating IS was determined by analyzing host urine and serum (Figure S3) samples. In contrast to Bt wild-type-colonized animals, Bt Δ1492-colonized mice had no detectable IS in urine or serum (Figure S3C) (limit of detection in serum 0.003 mg/dL). Data shown are means from five biological replicates. Error bars represent standard deviation. (B) When gnotobiotic mice colonized with a defined bacterial community were switched from a standard diet to a fructo-oligosaccharide (FOS) diet 28 days post-inoculation, a shift from a Bt (indole producer)-dominant to a B. caccae (indole non-producer)-dominant community was observed. Data shown are from five biological replicates. (C) Concomitant with this shift in community composition, the level of urinary IS decreased significantly from day 28 to day 35 (P = 0.0085). Asterisks represent a significant difference between groups, Welch’s t test, n = at least 3 per group. (D) Conventional mice were reprogrammed to accumulate near-zero levels of IS. Following three days of treatment with an antibiotic cocktail, mice were treated with erythromycin and colonized with either Bt wild-type + ErmR or Bt Δ1492 + ErmR (days 3–9). Colonization with Bt wild-type + ErmR resulted in higher IS levels (Day 5, P = 0.035, at least 9 biological replicates per group; Day 7, P = 0.002, at least 7 biological replicates per group; Day 9, P = 0.047, 4 biological replicates per group). Error bars represent standard deviation. Asterisks represent a significant difference between groups, Welch’s t test. IS levels in (A) and (B) were not normalized to creatinine because a diet change in these experiments ablated the effectiveness of using creatinine normalization to account for changes in hydration state of the animals.
