Abstract
Hypo- and hyperthyroid states, as well as functional abnormalities in the hypothalamic-pituitary-thyroid axis have been associated with psychiatric conditions like anxiety and depression. However, the nature of this relationship is poorly understood since it is difficult to ascertain the thyroid status of the brain in humans. Data from animal models indicate that the brain exhibits efficient homeostatic mechanisms that maintain local levels of the active thyroid hormone, triiodothyronine (T3) within a narrow range. To better understand the consequences of peripheral and central thyroid status for mood-related behaviors, we used a mouse model of type 3 deiodinase (DIO3) deficiency (Dio3 −/− mouse). This enzyme inactivates thyroid hormone and is highly expressed in the adult central nervous system. Adult Dio3 −/− mice exhibit elevated levels of T3-dependent gene expression in the brain, despite peripheral hypothyroidism as indicated by low circulating levels of thyroxine and T3. Dio3 −/− mice of both sexes exhibit hyperactivity and significantly decreased anxiety-like behavior, as measured by longer time spent in the open arms of the elevated plus maze and in the light area of the light/dark box. During the tail suspension, they stayed immobile for a significantly shorter time than their wild-type littermates, suggesting decreased depression-like behavior. These results indicate that increased thyroid hormone in the brain, not necessarily in peripheral tissues, correlates with hyperactivity and with decreases in anxiety and depression-like behaviors. Our results also underscore the importance of DIO3 as a determinant of behavior by locally regulating the brain levels of thyroid hormone.
Keywords: Dio3, hypothyroidism, hyperthyroidism, anxiety, depression, hyperactivity
Introduction
The relationship between thyroid hormone status and the susceptibility and outcome of psychiatric conditions, especially mood disorders, is not well understood. Although abnormal thyroid parameters in the serum have been associated with several of these conditions, findings may vary between different human studies and the nature of that relationship remains unclear (Dayan and Panicker, 2013; Fava et al., 1995; Haggerty and Prange, 1995).
Some human studies indicate that hypothyroidism is associated with an increased risk of anxiety, depression and suicide (Chueire et al., 2007; Cleare et al., 1995; Constant et al., 2005; Custro et al., 1994; Sinai et al., 2009). Hypothyroid patients with certain single nucleotide polymorphisms in the thyroid hormone transporter OATP1C1 are more susceptible to develop depression (van der Deure et al., 2008). In addition, abnormal parameters in thyroid function have been associated with postpartum depression risk (Albacar et al., 2010; Lucas et al., 2001; Pedersen et al., 2007; Plaza et al., 2010), and serum thyroid hormone concentrations have been reported to influence depression severity (Berent et al., 2014; Joffe and Marriott, 2000). Furthermore, altered levels of TSH in response to the administration of thyrotropin-releasing hormone (TRH) are also associated with depression severity or susceptibility (Duval et al., 1994; Kim et al., 2015; Kirkegaard and Faber, 1986; Targum et al., 1984), suggesting a relationship between the disorder and the setup of the hypothalamic-pituitary-thyroid (HPT) axis. Even the use of thyroid hormones, together with antidepressants, appears to improve outcomes (Bauer et al., 2002; Bauer et al., 2005; Joffe and Sokolov, 2000; Joffe et al., 1995; Prange, 1996), in some cases probably due to the influences of certain antidepressants on the HPT axis (Shelton et al., 1993) or the local availability of thyroid hormone in the brain regions (Baumgartner et al., 1994; Campos-Barros et al., 1994).
Although a majority of research supports an association between hypothyroidism and depression, the results from other studies do not align with this idea. Park et al. reported no association between thyroid disease and depression in older men (Park et al., 2010), Frye et al. found no correlation between depression and thyroid parameters in cerebrospinal fluid (Frye et al., 1999), and Medici et al. observed that an increased risk of depression is associated with low serum TSH (Medici et al., 2014). There might be significant differences in the experimental design and populations between these and other studies, but the significant inconsistency of findings has lead to the suggestion that thyroid tests offer misleading information for psychiatric patients (Lasser and Baldessarini, 1997).
Studies in animals largely confirm the occurrence of increased anxiety- and depression-like behaviors in rodent models of hypothyroidism (Darbra et al., 2003; Ge et al., 2014; Yu et al., 2015). These abnormal behaviors are observed in mice with a hypofunctional thyroid hormone receptor alpha1 (Buras et al., 2014), and are normalized with T3 treatment (Venero et al., 2005). The depression-related phenotypes observed in mice with an altered HPT axis (Shukla et al., 2010; Zeng et al., 2007), also supports its association with mood disorders. There are less published work on the psychiatric effects of excessive exposure to thyroid hormone, but hyperthyroidism has also been associated with mood alterations (Demet et al., 2002; Simon et al., 2002), suggesting that thyroid hormone action in the brain needs to be maintained at appropriate levels for normal behavioral outcomes.
One potential explanation for the disparity of some findings is the relatively loose relationship between thyroid hormone parameters in the serum and actual thyroid hormone signaling in the brain. The latter is greatly influenced, not as much by circulating levels of thyroid hormones, but by local determinants of thyroid hormone action, including thyroid hormone transporters and deiodinase enzymes (Bianco, 2011). When these factors are genetically altered in mouse models, a marked divergence may exist between the thyroid status of the serum and that of the brain (Bianco, 2011). We hypothesized that the thyroid hormone status of the brain is more consequential than that of the serum for mood-related behaviors that depend on thyroid hormones.
One critical determinant of thyroid hormone action in the central nervous system is the type 3 deiodinase (DIO3), an enzyme encoded by the imprinted gene Dio3 (Hernandez et al., 2002) that inactivates thyroid hormone (Hernandez, 2005). During adult life, Dio3 −/− mice exhibit systemic hypothyroidism due to functional deficits in the HPT axis, but marked brain thyrotoxicosis due to impaired T3 clearance in the central nervous system (Hernandez et al., 2010).
Here we use this mouse model of divergence between the peripheral and central thyroid state to better understand the relationship between mood disorders and thyroid hormone status in the brain versus that in the circulation. We find that Dio3 −/− mice exhibit hyperactivity and a reduction in anxiety- and depression-like behaviors despite their peripheral hypothyroidism. The new data shed additional light on the relationship between thyroid hormone status and mood disorders and underscores the potential relevance of DIO3 for these conditions.
2. Methods
2.1. Animals
Male (C57Bl/6J genetic background) and female (129/SvJ genetic background) mice were mated to generate the experimental animals used for behavioral analysis and gene expression. Both breeders were heterozygous for an inactivating mutation in the gene that codes for the D3 enzyme, Dio3 (Hernandez et al., 2006). Thus, the Dio3 +/+ and Dio3 −/− littermates so generated are on a defined 50:50 129/SvJ/C57Bl/6J mixed genetic background. The genomic heterozygosity of experimental animals reflects better the genetic variation in the human population and avoids extreme baseline behavior that may exist in inbred strains preventing the detection of a behavioral phenotype. All mice were kept in a 12 h light/dark cycle and fed regular chow ad libitum. Adult animals (4–5 month old) were sacrificed by CO2 asphyxiation. Mice carrying the FINDT3 transgene used for β-galactosidase staining of brain sections were also littermates but in a heterogeneous 129/SvJ/C57Bl/6J mixed genetic background. Brains from these mice were collected and processed for β-galactosidase staining of coronal section as previously described (Hernandez et al., 2010).
For the behavioral tests, two different cohorts of mice (Dartmouth cohort and the MMCRI cohort) were generated at different Institutions and tested at different facilities. One was generated at the Geisel School of Medicine at Dartmouth (Dartmouth cohort) and the other at MMCRI (MMCRI cohort). For the Dartmouth cohort, the testing schedule was as follows: open filed test at day one, elevated plus maze at day four, marble burying test at day 8, tail suspension test at day 15, and forced swimming test at day 22. For the MMCRI cohort, the testing regime was: light/dark box test at day one, elevated plus maze test at day four, elevated zero maze at day 8, and marble burying test at day 11. The shorter time intervals between tests is based on battery testing done at The Jackson Laboratory (O’Leary et al., 2013). A total of 103 mice were used in the behavioral studies. The Dartmouth cohort comprised 56 mice (15 Dio3 +/+ males, 14 Dio3 −/− males, 16 Dio3 +/+ females and 11 Dio3 −/− females), while the MMCRI cohort comprised 47 mice (17 Dio3 +/+ males, 9 Dio3 −/− males, 9 Dio3 +/+ females and 12 Dio3 −/− females). Not all mice were used in all tests. The number of mice from which data was obtained for a given test is indicated in the figure legends. The Institutional Animal Care and Use Committees of the Geisel School of Medicine at Dartmouth and the Maine Medical Center Research Institute approved all procedures and behavioral tests. The animal work was performed following the guidelines of the National Institute of Health Guide for the Care and Use of Laboratory Animals.
2.2. Quantitative real time RT-PCR (qRT-PCR)
The neocortex was harvested and frozen on dry ice, and total RNA was extracted using the RNeasy kit from Qiagen (Valencia, CA). Total RNA (1 μg) was reverse transcribed with M-MLV reverse transcriptase in the presence of random hexamers at 42 °C for 1 h. Reverse transcription reactions were diluted appropriately and aliquots were used as templates in duplicate real-time PCR reactions for each of the selected genes. Reactions were run in a 7300 RT PCR System (Applied Biosystems) using the SYBR® Select Master Mix from Applied Biosystems. Real time PCR reactions underwent an initial 10 minutes denaturing step, followed by 36 cycles of a denaturing step (94 °C for 30 seconds) and an annealing/extension step (60 °C for one minute). The sequence of the primers used was (5′ to 3′): Itih3, GCACGTTCAGTTGGCTAGAC and CCATCTCCAAAGGACACCAC; Hr, AGCACTGTGTGGCATGTGTT and AACCCTGCATCCAAGTAGCA; Rn18s, GGAGTATGGTTGCAAAGCTG and TCGCTCCACCAACTAAGAAC; Sema7a, AAGTGGTCGTTCACCGCATG and CCACCACCTTGTGAATGGTG; Aldh1a1, CCTTGCATTGTGTTTGCAGATG and GCTCGCTCAACACTCCTTTTC; Shh, GGACGTAAGTCCTTCACCAG and TTCTGTGAAAGCAGAGAACTCC; Htr2c, ACTTGTCATGCCCCTGTCTC and CCGCGAATTGAACCGGCTAT; Bdnf, TGCAGGGGCATAGACAAAAGG and CTTATGAATCGCCAGCCAATTCTC; Thra, CTTTGAACTGGGCAAGTCAC and TGGCCGCCTGAGGCTTTAGACTTC; Gapdh, AGGAGCGAGACCCCACTAAC and CGGAGATGATGACCCTTTTG; Actb, TGGGTATGGAATCCTGTGGC and CTGCATCCTGTCAGCAATGC. Expression data were read in an internal standard curve prepared by subsequent dilutions of a mix of aliquots of reverse transcription reactions from the samples in a given experiment. Expression of specific genes was corrected by the expression of a control gene (Rn18s, Actb or Gapdh). Data are expressed in arbitrary units.
2.3. Hormone determinations
Blood was taken from the descending vena cava. After coagulation at 4 °C for 4 h, serum was obtained by centrifugation and stored at −70 °C until analysis. Serum levels of thyroid hormones were determined in duplicate using the commercial Coat-a-Count Total T3 and Total T4 radioimmunoassay kits from Siemens USA (New York, NY). Brain T3 content was determined by non-equilibrium radioimmunoassay after tissue extraction as previously described (Galton et al., 2014).
2. 4. Open field test
Mice were housed in the testing room for two to three hours before the test and were left undisturbed during this period. We employed a 60 cm-long square field surrounded by a 15 cm-high walls. At the beginning of the test the mouse was placed in the center of the field and allowed to explore the maze freely while being video recorded for 5 minutes using a Videomex automated system (Columbus Instruments). The center area was defined as 50×50 cm2 and surrounded by a 10 cm wide periphery.
2. 5. Elevated plus and zero maze tests
Mice were housed in the testing room for two to three hours prior to the test and left undisturbed during this period. The tests were performed either at Dartmouth Medical School using the elevated plus maze (EPM) and the Viodeomex automated system (Columbus instruments), or at Maine Medical Center Research Institute using the EPM and elevated zero maze (EZM) and the ANY-maze™ video tracking system (Stoelting). The mazes are elevated and have a cross or annular shape. Opposing arms (EPM) or quadrants (EZM) are either enclosed by walls or open. Mice were placed in the center (EPM) or facing an enclosed quadrant (EZM) to start the test and recorder for 3 min (Dartmouth mice cohort) or 5 min (MMCRI mice cohort), while exploring the maze freely. Mice falling off the maze were excluded form the tests and analysis.
2. 6. Marble burying test
This test was performed essentially following the protocol described by Deacon (Deacon, 2006). In brief, mice were individually placed in a 20 × 35 cm2 (Dartmouth cohort) or 45 × 23 cm2 (MMCRI cohort) large cage that contained 10 cm of pressed pine shavings bedding, with 18 equally spaced glass marbles on top. Mice were allowed to explore the cage undisturbed and in low lighting for 30 min. Marbles are buried as an indirect result of the digging behavior of the mice. Marbles buried by at least two thirds of their height were counted as buried. Results are expressed as the percentage of buried marbles.
2. 7. Dark/Light box test
The light-dark box test is used to assess anxious behavior in mice. The arena measures 40×40 cm2 total and is surrounded by 35 cm high walls. The box is divided in half, using clear acrylic glass for the light area and black acrylic glass for the dark area including a top cover. The two areas are connected through an opening in the middle of the dividing wall allowing the animal to move freely from one half into the other. At the beginning of the test the animal is placed in the light arena with its back against the wall facing the opening to the dark area. The animal is allowed to explore the arena freely for 10 min while being recorded using the ANY-maze™ video-tracking system (Stoelting).
2. 8. Tail suspension test
Mice were housed in the testing room for two to three hours before the test and were undisturbed during this period. Mice caged together were tested in parallel. Using adhesive tape, mice were suspended by their tail from a shelf above a flat surface located approximately 15 cm from the mouse head. Mice were videotaped for 6 min. An investigator blind to the genotype of the mice analyzed the recordings and scored the latency to become immobile, the episodes of immobility, the duration of these episodes and the total time that the mice spent immobile during the test. No tail-climbing behavior was observed in any of the mice.
2. 9. Forced swimming test
The day before the test, animals were placed into a room adjacent to behavioral laboratory. Animals from the same cage were tested in parallel to avoid order effect. Mice were removed from their cages and placed in a 1.5 L beaker (d=13cm) filled with 28 °C water (to a height of 12.5–13 cm) for a six-minute period. The animals were video recorded for 6 min, and the video was analyzed and scored afterwards by a trained observer blind to the mice genotypes. The number and length of episodes of immobility were scored. Immobility is defined as the animal not moving or making just the necessary movements to stay afloat, as opposed to active swimming, during which the mouse is actively struggling in an effort to escape the water.
2. 10. Statistical Analysis
We used GraphPad Prism Software for all statistical analysis. Statistical significance was determined using two-way ANOVA for all the groups, followed by SIDAK’s test. Data from the two different mouse cohorts were analyzed separately.
3. Results
3.1. Serum levels of T4 and T3 and T3-dependent gene expression in cerebral cortex
Serum levels of T4 and T3 in adult Dio3 −/− mice of both sexes were significantly lower than those in Dio3 +/+ animals. Serum T4 and serum T3 were decreased approximately 40% and 20%, respectively, in Dio3 −/− mice of both sexes (Fig. 1A and 1B).
Fig. 1.
Serum and brain thyroid hormone status, and brain gene expression in Dio3 −/− mice. Serum levels of T3 and T4 (A and B). Brain T3 concentrations in adult males (C). Cortex gene expression of T3-regulated genes (D). Cortex genes expression of genes regulating T3 availability (Dio2) and signaling (Thra) (E). T3-dependent beta-galactosidase staining of cortex and hippocampus of 6 month old mice (F). Data represent the mean ± SEM of experiments with n= 6 to 12 mice per experimental group (A–E). *, **, ***, indicate P<0.05, P<0.01, P<0.001, respectively, as determined by two-way ANOVA followed by Sidak’s test.
Brain concentration of T3 was significantly increased in the brain of Dio3 −/− adult male mice compared to Dio3 +/+ mice (Fig. 1C), consistent with published observations (Galton et al., 2014). To assess the level of thyroid hormone signaling in the brain, we determined the cortical expression of several genes regulated in the brain by thyroid hormones (Chatonnet et al., 2015). These included hairless (Hr), brain-derived neurotrophic factor (Bdnf), serotonin receptor 2c (Htr2c), sonic hedgehog (Shh), semaphorin 7a (Sema7a), aldehyde dehydrogenase 1 family member a1 (Aldh1a1) and inter-alpha-trypsin inhibitor heavy chain 3 (Itih3). Compared to Dio3 +/+ male mice, the expression of these genes was elevated in Dio3 −/− males, the observation being statistically significant for most of them (Fig. 1D). Dio3 −/− female mice did not show an increased expression of these genes, except for Hr and Itih3. Expression of the thyroid hormone receptor alpha (Thra) was unchanged in the cerebral cortex of Dio3 −/− of either sex, while expression of the type 2 deiodinase gene (Dio2), which increases thyroid hormone action by generating T3 from T4, was reduced in Dio3 −/− females, but unchanged in Dio3 −/− males (Fig. 1E). In addition, β-galactosidase staining of brain sections of Dio3 +/+ and Dio3 −/− mice carrying a T3-regulated β-galactosidase transgene (Hernandez et al., 2010) further indicated abnormally elevated levels of thyroid hormone action in the brain of Dio3 −/− mice. Increases in β-galactosidase staining are apparent in the cortex and in the pyramidal cell layer of the hippocampus (Fig. 1E top and bottom, respectively).
These results are consistent with those previously published for Dio3 −/− mice in other genetic backgrounds (Hernandez et al., 2006; Hernandez et al., 2010), and demonstrate that the Dio3 −/− mice utilized in these studies manifest an excess of thyroid hormone in the brain despite the fact that their serum levels of thyroid hormones are lower than those in their wild type littermates.
3. 2. Exploratory, and anxiety- and depression-related behaviors
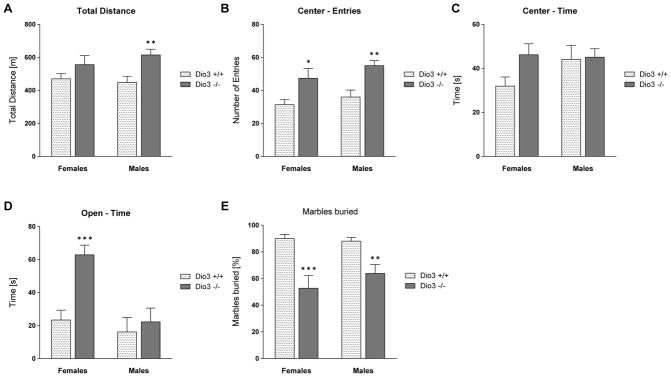
We investigated whether the enhanced TH action in the brain of Dio3 −/− mice is associated with changes in behavior. An initial cohort of mice was submitted to the open field (OF), elevated plus maze (EPM) and marble burying (MB) tests, the first two of them using a Videomex system (see Methods). During the OF test, Dio3 −/− mice traveled a significantly longer distance than their Dio3 +/+ littermates (Fig, 2A). This behavioral difference of Dio3 −/− mice was statistically significant in males, but not in females (Fig. 2A). Also, Dio3 −/− mice entered the center of the OF more often than Dio3 +/+ mice, and this behavior was observed in both males and females (Fig. 2B). Dio3 −/− females did spend significantly more time in the center area than Dio3 +/+ females, whereas no difference was observed in males (Fig. 2C). In the EPM test, Dio3 −/− female mice spent significantly more time in the open arms than the wild type littermates (Fig. 2D). This behavior was similar in Dio3 −/− males, but did not reach statistical significance (Fig. 2D). Marble burying, an indirect result of digging behavior, is associated with anxiety and repetitive behaviors (Deacon, 2006; Silverman et al., 2015), and was markedly diminished in Dio3 −/− mice of both sexes (Fig. 2E). The results of these studies show increased locomotion and exploratory behavior, and reduced anxiety-like behavior in Dio3 −/− mice.
Fig. 2.
Anxiety-like behavior in Dio3 −/− mice. Dio3 +/+ and Dio3 −/− mice (Dartmouth cohort) underwent several different behavioral tests to assess anxiety-like behavior. The total distance traveled in the open field test (OFT) is shown in (A), the number of entries into the center area in (B) and the time spent in the center in (C). (D) shows the time the mice spent in the open arms of the elevated plus maze (EPM), and (E) shows the percentage of buried marbles in the marble burying test. Animals used in the OFT and marble burying: Data represent the mean ± SEM of results from 6 to 16 mice (per genotype and sex) representing at least three different litters. *, **, ***, indicate P<0.05, P<0.01, P<0.001, respectively, Dio3 +/+ vs Dio3 −/−, as determined by two-way ANOVA followed by Sidak’s test.
A more extensive behavioral analysis of Dio3 −/− mice was performed in a different facility and institution, using a second cohort of mice and a different video-tracking system (see Methods). Animals underwent the EPM and the elevated zero maze (EZM) tests. Dio3 −/− mice entered significantly more often than Dio3 +/+ mice the open arms of the EPM (Fig. 3A) and the open quadrants of the EZM (Fig. 3B). This change in behavior was observed in Dio3 −/− mice of both sexes, although it was not statistically significant in the case of the number of entries of Dio3 −/− females into the open arms of the EPM (Fig. 3A). In addition, Dio3 −/− mice of both sexes spent more than twice as much time in the open area of both mazes than wild type mice (Fig. 3C&D). In both the EPM and the EZM, Dio3 −/− mice of both sexes also traveled a greater distance in total (Fig. 3E&F), and spent less time immobile (Fig. 3G&H).
Fig. 3.
Elevated plus maze & elevated zero maze. Dio3 +/+ and Dio3 −/− mice (MMCRI cohort) were tested in both the elevated plus maze (EPM) and the elevated zero maze (EZM). Data show the number of entries in the open arms (A & B) and the time spent there (C & D). Panels E & F show the total distance traveled in the maze as well as the total time the animals spent immobile (G & H). Data represent the mean ± SEM of results from 6 to 12 mice (per genotype and sex) representing at least three different litters. *, **, ***, indicate P<0.05, P<0.01, P<0.001, respectively, Dio3 +/+ vs Dio3 −/−, as determined by two-way ANOVA followed by Sidak’s test. For more detailed data see supplemental data.
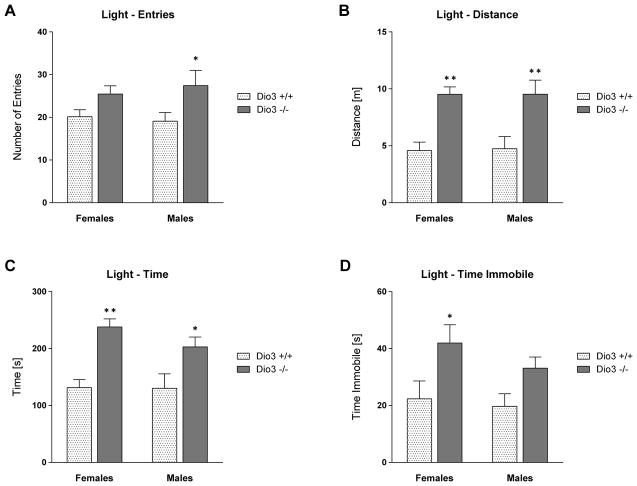
We also subjected the mice to the light/dark box (LDB) test. In this test, Dio3 −/− mice of both sexes entered the lit area more often than Dio3 +/+ mice (Fig. 4A), traveled a greater distance (Fig. 4B) and spent significantly more time in this zone (Fig. 4C). Despite traveling more distance, Dio3 −/− mice of both sexes were immobile in the lit area for a longer time, compared to Dio3 +/+ mice (Fig. 4D). This is because they moved significantly faster while the percentage of time spent immobile was not significantly different from that in wild type mice (Supplemental Data). Taken together, the results from the EPM, EZM and LDB tests indicate increased locomotion and reduced anxiety-like behavior in Dio3 −/− mice of both sexes.
Fig. 4.
Light & dark box. Dio3 +/+ and Dio3 −/− mice (MMCRI cohort) were tested in the light & dark box (LDB). Shown here are: the number of entries (A), the distance traveled (B), the total time (C) and the time spent immobile (D) in the light area. Data represent the mean ± SEM of results from 6 to 9 mice (per genotype and sex) representing at least three different litters. *, **, indicate P<0.05, P<0.01, respectively, Dio3 +/+ vs Dio3 −/−, as determined by two-way ANOVA followed by Sidak’s test. For more detailed data see supplemental data.
We subjected this second cohort of mice to the MB test. Consistent with the results obtained from the first cohort of mice (Fig. 2E), Dio3 −/− mice buried significantly less marbles (Fig. 5). The reduced marble burying behavior was observed in Dio3 −/− mice of both sexes, but did not achieve statistical significance in females (Fig. 5).
Fig. 5.

Marble burying test. Dio3 +/+ and Dio3 −/− (MMCRI cohort) mice were tested in the marble burying test. The percentages of marbles buried by at least 2/3 of their diameter are shown. Data represent the mean ± SEM of results from 6 to 9 mice (per genotype and sex) representing at least three different litters. ***, indicate P<0.05, P<0.01, P<0.001, respectively, Dio3 +/+ vs Dio3 −/−, as determined by two-way ANOVA followed by Sidak’s test.
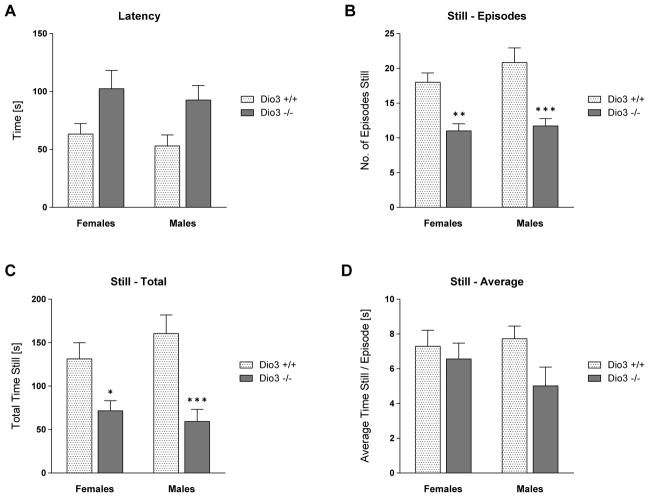
We also evaluated depression-like behaviors in Dio3 −/− mice using the tail suspension and the forced swim tests. The tail suspension test showed that the latency to immobility was significantly increased in Dio3 −/− mice, when compared to that in Dio3 +/+ mice (Fig. 6A). In addition, the number of episodes in which the animals were immobile as well as the total time spent immobile were markedly reduced in Dio3 −/− mice (Fig. 6B and 6C). The average length of the episodes in which the mice were immobile was not significantly different between genotypes (Fig. 6D).
Fig. 6.
Tail suspension test to assess depression-like behavior in Dio3 −/− mice. Dio3 +/+ and Dio3 −/− mice (Dartmouth cohort) were tested in the tail suspension test. The latency to immobility was measured (A). The number of episodes (B), the total time (C) and the average time per episode (D), when the mice remained still were analyzed. Data represent the mean ± SEM of results from 6 to 9 mice (per genotype and sex) representing at least three different litters. *, **, ***, indicate P<0.05, P<0.01, P<0.001, respectively, Dio3 +/+ vs Dio3 −/−, as determined by two-way ANOVA followed by Sidak’s test.
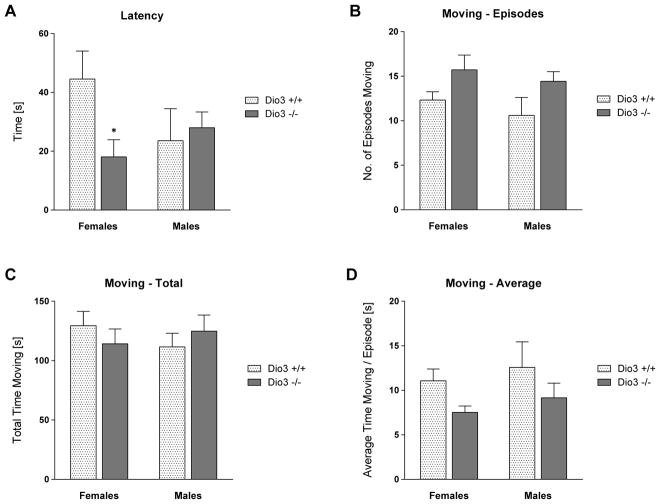
Results from the forced swimming test did not reveal marked differences between the behavior of Dio3 +/+ and Dio3 −/− mice, although some test parameters were distinct in Dio3 −/− mice (Fig. 7). Latency to immobility was reduced in Dio3 −/− females compared to that in Dio3 +/+ female littermates (Fig. 7A), but was not different males. The overall time spent moving was the same across genotypes and sexes (Fig. 7C). The moving episodes of Dio3 −/− mice were shorter, and occurred more often than in Dio3 +/+ mice (Fig. 7B and 7D), however none of these parameters reached statistical significance.
Fig. 7.
Forced swim test. Dio3 +/+ and Dio3 −/− mice (Dartmouth cohort) were tested in the forced swim test. (A), Latency to immobility. (B), Number of moving episodes. (C), Total moving time. (D) Average duration of moving episodes. Data represent the mean ± SEM of results from 6 to 9 mice (per genotype and sex) representing at least three different litters. *, indicates P<0.05, Dio3 +/+ vs Dio3 −/−, as determined by two-way ANOVA followed by Sidak’s test.
4. Discussion
The results from studies analyzing the relationship between thyroid hormone and mood disorders are sometimes inconsistent, since thyroid hormone status, as determined by hormone concentrations in the blood, may not necessarily reflect the true thyroid hormone state of the brain. To provide new insights on that relationship, we have evaluated behaviors relevant to mood disorders in a mouse model, the DIO3 deficient mouse, exhibiting hypothyroidism in peripheral tissues, but augmented thyroid hormone action in the central nervous system. Our data consistently demonstrate that DIO3 deficiency leads to decreased anxiety and depression-like behavior, and elevated locomotor activity. The fact that we obtained essentially the same results in two different mouse cohorts, while performing the experiments in different facilities, further underscores the robustness of this phenotype. These behavioral abnormalities of Dio3 −/− mice appear to be present in both males and females. The difference in the absolute values obtained in the MB tests between the first and second animal cohorts largely reflects the different size of the arena used in the tests.
The expression level of Dio3 is high in the brain, mostly in neurons, and its biochemical function is to inactivate thyroid hormones. Thus, the behavioral impact of Dio3 deficiency is likely the result of increased thyroid hormone signaling in multiple regions of the central nervous system, an observation that has been shown in previous work (Hernandez et al., 2010) and is further confirmed here. Those regions include the hippocampus and the amygdala, which are particularly involved in anxiety and depression-like behaviors. Our behavioral observations in DIO3 deficiency are consistent with the phenotype observed in a mouse model with reduced thyroid hormone action in the brain as a result of a mutant, functionally impaired thyroid hormone receptor alpha (Venero et al., 2005; Wallis et al., 2008). These mice, in contrast to Dio3 −/− mice, have reduced levels of thyroid hormone signaling, and manifest increased anxiety and poor locomotor activity.
Since thyroid hormones exert broad effects in the developing and mature central nervous system, it is difficult to pinpoint the specific neural systems that are affected by elevated local levels of thyroid hormone and ultimately determine behavior. In this regard, thyroid hormone increases serotonin receptor activity and serotonergic response (Gur et al., 2004; Mason et al., 1987), processes that are targeted by certain antidepressants and anxiolytics. Drugs that modify serotonin transmission also inhibit marble-burying behavior (Deacon, 2006; Njung’e and Handley, 1991a, b). Thus it is possible that altered serotoninergic systems in Dio3 −/− mice are contributing to their behavior, as suggested by the abnormal expression of the gene encoding serotonin receptor 2C. The altered expression of Bndf and Itih3, two genes linked to neurodevelopmental and psychiatric conditions (Craddock et al., 2005; Lotan et al., 2014) also support an important role for DIO3 in the central regulation of thyroid hormone action.
The behavioral phenotype of Dio3 −/− mice is robust for both males and females, although we observed some modest differences between sexes. Interestingly, our gene expression data also suggest a sexual dimorphism at the molecular level, as Dio3 −/− males show more extensive differences in T3-dependent gene expression than females. In addition, although expression of thyroid hormone receptor is not altered in DIO3-defient mice, the expression of Dio2, the deiodinase that locally produces T3 from T4 thyroid hormone action, is decreased in females but not in males. These observations suggest a sexually dimorphic molecular signature concerning thyroid hormone signaling in the brain of Dio3 −/− mice. This is not entirely surprising based on published evidence linking sex-steroid hormones with Dio3 and thyroid hormone signaling. These observations include the regulation of DIO3 by estrogen (Wasco et al., 2003), and the transient but very high expression of Dio3 in specific areas of the neonatal rat brain (Escamez et al., 1999) that are enriched in androgen and estrogen receptors (DonCarlos, 1996) and involved in brain sexual differentiation. In addition, the regulation of gene expression in the brain is susceptible to crosstalk between thyroid hormone and estrogen receptors (Vasudevan et al., 2002; Vasudevan et al., 2001). Thus, it is likely that the enhanced thyroid hormone availability that occurs in the Dio3 −/− brain is affecting differently males and females.
Although the behavioral abnormalities in Dio3 −/− mice may result from increased thyroid hormone action in the adult brain, it is possible that they arise from altered development, since Dio3 −/− mice exhibit brain thyrotoxicosis also during development (Hernandez et al., 2006). This idea is supported by the observation that the administration of pharmacological doses of thyroid hormones during perinatal life results in abnormalities in the adult brain that include changes in serotonin and N-methyl-D-aspartate binding sites (Roskoden et al., 2002) and enhanced thyroid hormone action in the amygdala (Shukla et al., 2010).
The remarkable divergence in Dio3 −/− mice between the hypothyroid state of the serum and the hyperthyroid state of the brain provides an additional, important insight into the controversial association of mood disorders and altered thyroid hormone status. A similar divergence is shown in a rat model of prenatal thyroxine administration (Shukla et al., 2010). In this model, different thyroid hormone status between the amygdala and the circulation are associated with altered anxiety-like behavior.
Sexually dimorphic abnormalities in locomotor and stereotypic behavior have been reported in mice treated with L-carnitine (Benvenga et al., 2011), a compound that can inhibit the nuclear uptake of thyroid hormone (Benvenga et al., 2000). Although carnitine can modify the action of sex-steroids and glucocorticoids (Benvenga et al., 2011) and may have systemic effects in such a model, this observation supports a role of brain T3 signaling in the regulation of locomotor activity.
Regarding the hyperactive phenotype of Dio3 −/− mice, an intriguing, alternative interpretation of the data is that their behavioral profile reflects aspects of attention deficit and hyperactive disorder (ADHD). The hyperactivity and decreased anxiety may be a sign of greater risk-taking and more impulsive responses. In the anxiety tests, the increased time spent in the open arms of an elevated plus maze or the center region of an open field suggests a loss of typical cautionary avoidance of novel open areas. Decreased anxiety-like behavior and more risky exploratory responses have been observed in mouse models for mania and impulsivity (Matzel et al., 2008; van Enkhuizen et al., 2014). In this interpretation, the lower levels of immobility in the tail suspension test could be attributed to generally higher activity of Dio3 −/− mice, leading to more kicking and struggling. Interestingly, decreased marble-burying has been observed in several mouse models of developmental disorders (Balemans et al., 2010; DeLorey et al., 2011; Feyder et al., 2010; Moy et al., 2014), which might be due to decreased attention to complex elements of the environment. Since Dio3 −/− mice are also exposed to elevated levels of thyroid hormone during perinatal life (Hernandez et al., 2006), the similarities of Dio3 −/− mice with ADHD characteristics are consistent with the recent observation in humans of increased prevalence of ADHD in children that were exposed to increased levels of thyroid hormone during development (Andersen et al., 2014). A more recent study adds to the potential relationship between thyroid status during development and risk of ADHD, although it shows the opposite: an increase in ADHD symptoms in children born to mothers with hypothyroxinemia in early pregnancy (Modesto et al., 2015). Increased locomotion has also been observed in a rat model of transient neonatal hypothyroidism (Negishi et al., 2005). It is possible that the underlying ADHD pathophysiology may be affected by both low and high thyroid hormone status depending on the particular developmental stage, as it is well established that normal neurological outcomes require that the thyroid hormone status of the brain is maintained within a narrow range in a developmentally appropriate fashion. For instance, a deficit in either DIO2 or DIO3 leads to cochlear defects and impaired hearing (Ng et al., 2004; Ng et al., 2009), even though those deficits lead to opposite effects (decrease or increase, respectively) in thyroid hormone signaling. Another possibility is that serum thyroid hormone parameters do not reflect necessarily the thyroid status of the brain, as we underscore in the present work. Thus, maternal hypothyroxinemia may reflect enhanced thyroid hormone action in the brain due to genetic or epigenetic factors that may also apply to the developing fetus.
Our results showing increased brain thyroid hormone action and behavioral changes as a result of alterations in Dio3 expression may have clinical relevance. Since Dio3 is an imprinted gene preferentially expressed from the paternal allele in the mouse (Hernandez et al., 2002; Tsai et al., 2002), parental genetic effects and epigenetic alterations can modify its level of expression, ultimately affecting thyroid hormone action in the brain in region-specific manner (Martinez et al., 2014), with potential consequences for behavior. Behavioral abnormalities have been shown in genetic and epigenetic rat models of altered Dio3 dosage (Sittig et al., 2011a; Sittig et al., 2011b). These observations raise the possibility that epigenetic alterations in the regulation of DIO3 in humans may contribute to mood disorders by exerting changes in local thyroid hormone levels. This possibility is further supported by our recent observation that the human DIO3 is also subject to genomic imprinting (Martinez et al., 2016).
In summary, our results demonstrate a relationship between brain TH action and hyperactivity and anxiety and depression-like behaviors. DIO3 deficient mice with increased levels of thyroid hormone in the central nervous system exhibit altered anxiety and depressive-like behaviors, even while circulating levels of hormone indicate a hypothyroid state. Additional work is needed to define the regions of the brain contributing to these behaviors. A mouse model of DIO3 deficiency targeted to specific brain regions will be useful to this aim and to further investigate the link between thyroid hormones and mood and neurodevelopmental disorders. This link will introduce the possibility of manipulating thyroid hormone action in the brain for therapeutic purposes.
Supplementary Material
Highlights.
Decreased anxiety- and depression-like behaviors are associated with increased thyroid hormone action in the brain
Thyroid hormone action in the neonatal and/or adult brain leads to hyperactivity
The type 3 deiodinase deficient mouse resembles some aspects of Attention Deficit and Hyperactive Disorder
Acknowledgments
Role of funding sources
This work was partially supported by grants, MH083220, DK095908 and MH096050 from the National Institute of Diabetes, Digestive and kidney Diseases and from the National Institute of Mental Health.
We would like to thank Sheryl Moy for critically revising the manuscript.
Footnotes
Conflict of Interest
The authors have nothing to disclose
Disclosure
The authors declare no potential conflict of interest
Author’s Contributions: All authors participated in the design of the study, the acquisition and analysis of data, drafting results or more parts of the manuscript. All authors have approved the final version of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albacar G, Sans T, Martin-Santos R, Garcia-Esteve L, Guillamat R, Sanjuan J, Canellas F, Carot JM, Gratacos M, Bosch J, Gaviria A, Labad A, Zotes AG, Vilella E. Thyroid function 48h after delivery as a marker for subsequent postpartum depression. Psychoneuroendocrinology. 2010;35:738–742. doi: 10.1016/j.psyneuen.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Laurberg P, Wu CS, Olsen J. Attention deficit hyperactivity disorder and autism spectrum disorder in children born to mothers with thyroid dysfunction: a Danish nationwide cohort study. BJOG: an international journal of obstetrics and gynaecology. 2014;121:1365–1374. doi: 10.1111/1471-0528.12681. [DOI] [PubMed] [Google Scholar]
- Balemans MC, Huibers MM, Eikelenboom NW, Kuipers AJ, van Summeren RC, Pijpers MM, Tachibana M, Shinkai Y, van Bokhoven H, Van der Zee CE. Reduced exploration, increased anxiety, and altered social behavior: Autistic-like features of euchromatin histone methyltransferase 1 heterozygous knockout mice. Behavioural Brain Research. 2010;208:47–55. doi: 10.1016/j.bbr.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Bauer M, Baur H, Berghofer A, Strohle A, Hellweg R, Muller-Oerlinghausen B, Baumgartner A. Effects of supraphysiological thyroxine administration in healthy controls and patients with depressive disorders. Journal of Affective Disorders. 2002;68:285–294. doi: 10.1016/s0165-0327(00)00363-3. [DOI] [PubMed] [Google Scholar]
- Bauer M, London ED, Rasgon N, Berman SM, Frye MA, Altshuler LL, Mandelkern MA, Bramen J, Voytek B, Woods R, Mazziotta JC, Whybrow PC. Supraphysiological doses of levothyroxine alter regional cerebral metabolism and improve mood in bipolar depression. Molecular Psychiatry. 2005;10:456–469. doi: 10.1038/sj.mp.4001647. [DOI] [PubMed] [Google Scholar]
- Baumgartner A, Campos-Barros A, Gaio U, Hessenius C, Flechner A, Meinhold H. Carbamazepine affects triiodothyronine production and metabolization in rat hippocampus. Life Sci. 1994;54:401–407. doi: 10.1016/0024-3205(94)90123-6. [DOI] [PubMed] [Google Scholar]
- Benvenga S, Itri E, Hauser P, De Tolla L, Yu SF, Testa G, Pappalardo MA, Trimarchi F, Amato A. Gender differences in locomotor and stereotypic behavior associated with l-carnitine treatment in mice. Gender medicine. 2011;8:1–13. doi: 10.1016/j.genm.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Benvenga S, Lakshmanan M, Trimarchi F. Carnitine is a naturally occurring inhibitor of thyroid hormone nuclear uptake. Thyroid. 2000;10:1043–1050. doi: 10.1089/thy.2000.10.1043. [DOI] [PubMed] [Google Scholar]
- Berent D, Zboralski K, Orzechowska A, Galecki P. Thyroid hormones association with depression severity and clinical outcome in patients with major depressive disorder. Molecular biology reports. 2014;41:2419–2425. doi: 10.1007/s11033-014-3097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco AC. Minireview: cracking the metabolic code for thyroid hormone signaling. Endocrinology. 2011;152:3306–3311. doi: 10.1210/en.2011-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buras A, Battle L, Landers E, Nguyen T, Vasudevan N. Thyroid hormones regulate anxiety in the male mouse. Hormones and behavior. 2014;65:88–96. doi: 10.1016/j.yhbeh.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Campos-Barros A, Meinhold H, Stula M, Muller F, Kohler R, Eravci M, Putzien O, Baumgartner A. The influence of desipramine on thyroid hormone metabolism in rat brain. J Pharm Exp Ther. 1994;268:1143–1152. [PubMed] [Google Scholar]
- Chatonnet F, Flamant F, Morte B. A temporary compendium of thyroid hormone target genes in brain. Biochim Biophys Acta. 2015;1849:122–129. doi: 10.1016/j.bbagrm.2014.05.023. [DOI] [PubMed] [Google Scholar]
- Chueire VB, Romaldini JH, Ward LS. Subclinical hypothyroidism increases the risk for depression in the elderly. Archives of gerontology and geriatrics. 2007;44:21–28. doi: 10.1016/j.archger.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Cleare AJ, McGregor A, O’Keane V. Neuroendocrine evidence for an association between hypothyroidism, reduced central 5-HT activity and depression. Clin Endocrinol (Oxf) 1995;43:713–719. doi: 10.1111/j.1365-2265.1995.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Constant EL, Adam S, Seron X, Bruyer R, Seghers A, Daumerie C. Anxiety and depression, attention, and executive functions in hypothyroidism. Journal of the International Neuropsychological Society: JINS. 2005;11:535–544. doi: 10.1017/S1355617705050642. [DOI] [PubMed] [Google Scholar]
- Craddock N, O’Donovan MC, Owen MJ. The genetics of schizophrenia and bipolar disorder: dissecting psychosis. Journal of Medical Genetics. 2005;42:193–204. doi: 10.1136/jmg.2005.030718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custro N, Scafidi V, Lo Baido R, Nastri L, Abbate G, Cuffaro MP, Gallo S, Vienna G, Notarbartolo A. Subclinical hypothyroidism resulting from autoimmune thyroiditis in female patients with endogenous depression. J Endocrinol Invest. 1994;17:641–646. doi: 10.1007/BF03349679. [DOI] [PubMed] [Google Scholar]
- Darbra S, Garau A, Balada F, Sala J, Marti-Carbonell MA. Perinatal hypothyroidism effects on neuromotor competence, novelty-directed exploratory and anxiety-related behaviour and learning in rats. Behavioural brain research. 2003;143:209–215. doi: 10.1016/s0166-4328(03)00041-x. [DOI] [PubMed] [Google Scholar]
- Dayan CM, Panicker V. Hypothyroidism and depression. European thyroid journal. 2013;2:168–179. doi: 10.1159/000353777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon RMJ. Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nature Protocols. 2006;1:122–124. doi: 10.1038/nprot.2006.20. [DOI] [PubMed] [Google Scholar]
- DeLorey TM, Sahbaie P, Hashemi E, Li WW, Salehi A, Clark DJ. Somatosensory and sensorimotor consequences associated with the heterozygous disruption of the autism candidate gene, Gabrb3. Behavioural brain research. 2011;216:36–45. doi: 10.1016/j.bbr.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demet MM, Ozmen B, Deveci A, Boyvada S, Adiguzel H, Aydemir O. Depression and anxiety in hyperthyroidism. Archives of medical research. 2002;33:552–556. doi: 10.1016/s0188-4409(02)00410-1. [DOI] [PubMed] [Google Scholar]
- DonCarlos LL. Developmental profile and regulation of estrogen receptor (ER) mRNA expression in the preoptic area of prenatal rats. Brain Research Developmental Brain Research. 1996;94:224–233. doi: 10.1016/0165-3806(96)00067-3. [DOI] [PubMed] [Google Scholar]
- Duval F, Mokrani MC, Crocq MA, Bailey P, Macher JP. Influence of thyroid hormones on morning and evening TSH response to TRH in major depression. Biological psychiatry. 1994;35:926–934. doi: 10.1016/0006-3223(94)91239-4. [DOI] [PubMed] [Google Scholar]
- Escamez MJ, Guadano-Ferraz A, Cuadrado A, Bernal J. Type 3 iodothyronine deiodinase is selectively expressed in areas related to sexual differentiation in the newborn rat brain. Endocrinology. 1999;140:5443–5446. doi: 10.1210/endo.140.11.7244. [DOI] [PubMed] [Google Scholar]
- Fava M, Labbate LA, Abraham ME, Rosenbaum JF. Hypothyroidism and hyperthyroidism in major depression revisited. The Journal of clinical psychiatry. 1995;56:186–192. [PubMed] [Google Scholar]
- Feyder M, Karlsson RM, Mathur P, Lyman M, Bock R, Momenan R, Munasinghe J, Scattoni ML, Ihne J, Camp M, Graybeal C, Strathdee D, Begg A, Alvarez VA, Kirsch P, Rietschel M, Cichon S, Walter H, Meyer-Lindenberg A, Grant SG, Holmes A. Association of mouse Dlg4 (PSD-95) gene deletion and human DLG4 gene variation with phenotypes relevant to autism spectrum disorders and Williams’ syndrome. American Journal of Psychiatry. 2010;167(12):1508–17. doi: 10.1176/appi.ajp.2010.10040484. 2010 Dec. 67, 1508–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye MA, Dunn RT, Gary KA, Kimbrell TA, Callahan AM, Luckenbaugh DA, Cora-Locatelli G, Vanderham E, Winokur A, Post RM. Lack of correlation between cerebrospinal fluid thyrotropin-releasing hormone (TRH) and TRH-stimulated thyroid-stimulating hormone in patients with depression. Biological psychiatry. 1999;45:1049–1052. doi: 10.1016/s0006-3223(98)00322-9. [DOI] [PubMed] [Google Scholar]
- Galton VA, de Waard E, Parlow AF, St Germain DL, Hernandez A. Life without the iodothyronine deiodinases. Endocrinology. 2014;155:4081–4087. doi: 10.1210/en.2014-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge JF, Peng YY, Qi CC, Chen FH, Zhou JN. Depression-like behavior in subclinical hypothyroidism rat induced by hemi-thyroid electrocauterization. Endocrine. 2014;45:430–438. doi: 10.1007/s12020-013-0001-4. [DOI] [PubMed] [Google Scholar]
- Gur E, Lifschytz T, Van de Kar LD, Lerer B, Newman ME. Effects of triiodothyronine on 5-HT1a and 5-HT1b autoreceptor activity, and postsynaptic 5-HT1a receptor activity, in rat hypothalamus: lack of interaction with imipramine. Psychoneuroendocrinology. 2004;29:1172–1183. doi: 10.1016/j.psyneuen.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Haggerty JJ, Jr, Prange AJ., Jr Borderline hypothyroidism and depression. Annual review of medicine. 1995;46:37–46. doi: 10.1146/annurev.med.46.1.37. [DOI] [PubMed] [Google Scholar]
- Hernandez A. Structure and function of the type 3 deiodinase gene. Thyroid. 2005;15:865–874. doi: 10.1089/thy.2005.15.865. [DOI] [PubMed] [Google Scholar]
- Hernandez A, Fiering S, Martinez E, Galton VA, St Germain D. The gene locus encoding iodothyronine deiodinase type 3 (Dio3) is imprinted in the fetus and expresses antisense transcripts. Endocrinology. 2002;143:4483–4486. doi: 10.1210/en.2002-220800. [DOI] [PubMed] [Google Scholar]
- Hernandez A, Martinez ME, Fiering S, Galton VA, St Germain D. Type 3 deiodinase is critical for the maturation and function of the thyroid axis. Journal of Clinical Investigation. 2006;116:476–484. doi: 10.1172/JCI26240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez A, Quignodon L, Martinez ME, Flamant F, St Germain DL. Type 3 deiodinase deficiency causes spatial an temporal alterations in brain T3 signaling that are dissociated from serum thyroid hormone levels. Endocrinology. 2010;151:5550–5558. doi: 10.1210/en.2010-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe RT, Marriott M. Thyroid hormone levels and recurrence of major depression. The American journal of psychiatry. 2000;157:1689–1691. doi: 10.1176/appi.ajp.157.10.1689. [DOI] [PubMed] [Google Scholar]
- Joffe RT, Sokolov ST. Thyroid hormone treatment of primary unipolar depression: a review. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2000;3:143–147. doi: 10.1017/S146114570000184X. [DOI] [PubMed] [Google Scholar]
- Joffe RT, Sokolov ST, Singer W. Thyroid hormone treatment of depression. Thyroid. 1995;5:235–239. doi: 10.1089/thy.1995.5.235. [DOI] [PubMed] [Google Scholar]
- Kim EY, Kim SH, Rhee SJ, Huh I, Ha K, Kim J, Chang JS, Yoon DH, Park T, Ahn YM. Relationship between thyroid-stimulating hormone levels and risk of depression among the general population with normal free T4 levels. Psychoneuroendocrinology. 2015;58:114–119. doi: 10.1016/j.psyneuen.2015.04.016. [DOI] [PubMed] [Google Scholar]
- Kirkegaard C, Faber J. Influence of free thyroid hormone levels on the TSH response to TRH in endogenous depression. Psychoneuroendocrinology. 1986;11:491–497. doi: 10.1016/0306-4530(86)90009-0. [DOI] [PubMed] [Google Scholar]
- Lasser RA, Baldessarini RJ. Thyroid hormones in depressive disorders: a reappraisal of clinical utility. Harvard review of psychiatry. 1997;4:291–305. doi: 10.3109/10673229709030557. [DOI] [PubMed] [Google Scholar]
- Lotan A, Fenckova M, Bralten J, Alttoa A, Dixson L, Williams RW, van der Voet M. Neuroinformatic analyses of common and distinct genetic components associated with major neuropsychiatric disorders. Frontiers in neuroscience. 2014;8:331. doi: 10.3389/fnins.2014.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas A, Pizarro E, Granada ML, Salinas I, Sanmarti A. Postpartum thyroid dysfunction and postpartum depression: are they two linked disorders? Clin Endocrinol (Oxf) 2001;55:809–814. doi: 10.1046/j.1365-2265.2001.01421.x. [DOI] [PubMed] [Google Scholar]
- Martinez ME, Charalambous M, Saferali A, Fiering S, Naoumova A, St Germain DL, Ferguson-Smith A, Hernandez A. Genomic Imprinting Variations in the Mouse Type 3 Deiodinase Gene Between Tissues and Brain Regions. Mol Endocrinol. 2014;28:1875–1886. doi: 10.1210/me.2014-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez ME, Cox DF, Youth BP, Hernandez A. Genomic imprinting of DIO3, a candidate gene for the syndrome associated with human uniparental disomy of chromosome 14. Eur J Hum Genet. 2016 doi: 10.1038/ejhg.2016.66. advance online publication, June 22, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason GA, Bondy SC, Nemeroff CB, Walker CH, Prange AJ., Jr The effects of thyroid state on beta-adrenergic and serotonergic receptors in rat brain. Psychoneuroendocrinology. 1987;12:261–270. doi: 10.1016/0306-4530(87)90050-3. [DOI] [PubMed] [Google Scholar]
- Matzel LD, Babiarz J, Townsend DA, Grossman HC, Grumet M. Neuronal cell adhesion molecule deletion induces a cognitive and behavioral phenotype reflective of impulsivity. Genes, brain, and behavior. 2008;7:470–480. doi: 10.1111/j.1601-183X.2007.00382.x. [DOI] [PubMed] [Google Scholar]
- Medici M, Direk N, Visser WE, Korevaar TI, Hofman A, Visser TJ, Tiemeier H, Peeters RP. Thyroid function within the normal range and the risk of depression: a population-based cohort study. J Clin Endocrinol Metab. 2014;99:1213–1219. doi: 10.1210/jc.2013-3589. [DOI] [PubMed] [Google Scholar]
- Modesto T, Tiemeier H, Peeters RP, Jaddoe VW, Hofman A, Verhulst FC, Ghassabian A. Maternal Mild Thyroid Hormone Insufficiency in Early Pregnancy and Attention-Deficit/Hyperactivity Disorder Symptoms in Children. JAMA pediatrics. 2015;169:838–845. doi: 10.1001/jamapediatrics.2015.0498. [DOI] [PubMed] [Google Scholar]
- Moy SS, Riddick NV, Nikolova VD, Teng BL, Agster KL, Nonneman RJ, Young NB, Baker LK, Nadler JJ, Bodfish JW. Repetitive behavior profile and supersensitivity to amphetamine in the C58/J mouse model of autism. Behavioural brain research. 2014;259:200–214. doi: 10.1016/j.bbr.2013.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi T, Kawasaki K, Sekiguchi S, Ishii Y, Kyuwa S, Kuroda Y, Yoshikawa Y. Attention-deficit and hyperactive neurobehavioural characteristics induced by perinatal hypothyroidism in rats. Behavioural brain research. 2005;159:323–331. doi: 10.1016/j.bbr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Ng L, Goodyear RJ, Woods CA, Schneider MJ, Diamond E, Richardson GP, Kelley MW, St Germain D, Galton VA, Forrest D. Hearing loss and retarded cochlear development in mice lacking type 2 iodothyronine deiodinase. Proc Natl Acad Sci U S A. 2004;101:3474–3479. doi: 10.1073/pnas.0307402101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L, Hernandez A, He W, Ren T, Srinivas M, Ma M, Galton VA, St Germain DL, Forrest D. A protective role for type 3 deiodinase, a thyroid hormone-inactivating enzyme, in cochlear development and auditory function. Endocrinology. 2009;150:1952–1960. doi: 10.1210/en.2008-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njung’e K, Handley SL. Effects of 5-HT uptake inhibitors, agonists and antagonists on the burying of harmless objects by mice; a putative test for anxiolytic agents. British Journal of Pharmacology. 1991a;104:105–112. doi: 10.1111/j.1476-5381.1991.tb12392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njung’e K, Handley SL. Evaluation of marble-burying behavior as a model of anxiety. Pharmacology, Biochemistry & Behavior. 1991b;38:63–67. doi: 10.1016/0091-3057(91)90590-x. [DOI] [PubMed] [Google Scholar]
- O’Leary TP, Gunn RK, Brown RE. What are we measuring when we test strain differences in anxiety in mice? Behav Genet. 2013;43:34–50. doi: 10.1007/s10519-012-9572-8. [DOI] [PubMed] [Google Scholar]
- Park YJ, Lee EJ, Lee YJ, Choi SH, Park JH, Lee SB, Lim S, Lee WW, Jang HC, Cho BY, Woo JI, Kim KW. Subclinical hypothyroidism (SCH) is not associated with metabolic derangement, cognitive impairment, depression or poor quality of life (QoL) in elderly subjects. Archives of gerontology and geriatrics. 2010;50:e68–73. doi: 10.1016/j.archger.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Johnson JL, Silva S, Bunevicius R, Meltzer-Brody S, Hamer RM, Leserman J. Antenatal thyroid correlates of postpartum depression. Psychoneuroendocrinology. 2007;32:235–245. doi: 10.1016/j.psyneuen.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Plaza A, Garcia-Esteve L, Ascaso C, Navarro P, Gelabert E, Halperin I, Valdes M, Martin-Santos R. Childhood sexual abuse and hypothalamus-pituitary-thyroid axis in postpartum major depression. Journal of affective disorders. 2010;122:159–163. doi: 10.1016/j.jad.2009.07.021. [DOI] [PubMed] [Google Scholar]
- Prange AJ., Jr Novel uses of thyroid hormones in patients with affective disorders. Thyroid. 1996;6:537–543. doi: 10.1089/thy.1996.6.537. [DOI] [PubMed] [Google Scholar]
- Roskoden T, Zilles K, Schleicher A, Schwegler H. Transient postnatal thyroxine treatment leads to variation in transmitter binding site densities in the hippocampus of rats. Neuroscience Letters. 2002;333:21–24. doi: 10.1016/s0304-3940(02)00963-1. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Winn S, Ekhatore N, Loosen PT. The effects of antidepressants on the thyroid axis in depression. Biological psychiatry. 1993;33:120–126. doi: 10.1016/0006-3223(93)90311-z. [DOI] [PubMed] [Google Scholar]
- Shukla PK, Sittig LJ, Andrus BM, Schaffer DJ, Batra KK, Redei EE. Prenatal thyroxine treatment disparately affects peripheral and amygdala thyroid hormone levels. Psychoneuroendocrinology. 2010;35:791–797. doi: 10.1016/j.psyneuen.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Pride MC, Hayes JE, Puhger KR, Butler-Struben HM, Baker S, Crawley JN. GABAB Receptor Agonist R-Baclofen Reverses Social Deficits and Reduces Repetitive Behavior in Two Mouse Models of Autism. Neuropsychopharmacology. 2015;40:2228–2239. doi: 10.1038/npp.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NM, Blacker D, Korbly NB, Sharma SG, Worthington JJ, Otto MW, Pollack MH. Hypothyroidism and hyperthyroidism in anxiety disorders revisited: new data and literature review. Journal of affective disorders. 2002;69:209–217. doi: 10.1016/s0165-0327(01)00378-0. [DOI] [PubMed] [Google Scholar]
- Sinai C, Hirvikoski T, Dencker Vansvik E, Nordstrom AL, Linder J, Nordstrom P, Jokinen J. Thyroid hormones and personality traits in attempted suicide. Psychoneuroendocrinology. 2009;34:1526–1532. doi: 10.1016/j.psyneuen.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Sittig LJ, Herzing LB, Shukla PK, Redei EE. Parent-of-origin allelic contributions to deiodinase-3 expression elicit localized hyperthyroid milieu in the hippocampus. Molecular psychiatry. 2011a;16:786–787. doi: 10.1038/mp.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittig LJ, Shukla PK, Herzing LB, Redei EE. Strain-specific vulnerability to alcohol exposure in utero via hippocampal parent-of-origin expression of deiodinase-III. FASEB Journal. 2011b;25:2313–2324. doi: 10.1096/fj.10-179234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targum SD, Greenberg RD, Harmon RL, Kessler K, Salerian AJ, Fram DH. The TRH test and thyroid hormone in refractory depression. The American journal of psychiatry. 1984;141:463. doi: 10.1176/ajp.141.3.463a. [DOI] [PubMed] [Google Scholar]
- Tsai C, Lin SP, Ito M, Takagi NST, Ferguson-Smith AC. Genomic Imprinting contributes to thyroid hormone metabolism in the mouse embryo. Current Biology. 2002;12:1221–1226. doi: 10.1016/s0960-9822(02)00951-x. [DOI] [PubMed] [Google Scholar]
- van der Deure WM, Appelhof BC, Peeters RP, Wiersinga WM, Wekking EM, Huyser J, Schene AH, Tijssen JG, Hoogendijk WJ, Visser TJ, Fliers E. Polymorphisms in the brain-specific thyroid hormone transporter OATP1C1 are associated with fatigue and depression in hypothyroid patients. Clin Endocrinol (Oxf) 2008;69:804–811. doi: 10.1111/j.1365-2265.2008.03267.x. [DOI] [PubMed] [Google Scholar]
- van Enkhuizen J, Geyer MA, Halberstadt AL, Zhuang X, Young JW. Dopamine depletion attenuates some behavioral abnormalities in a hyperdopaminergic mouse model of bipolar disorder. Journal of affective disorders. 2014;155:247–254. doi: 10.1016/j.jad.2013.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan N, Kia HK, Inoue S, Muramatsu M, Pfaff D. Isoform specificity for oestrogen receptor and thyroid hormone receptor genes and their interactions on the NR2D gene promoter. Journal of Neuroendocrinology. 2002;14:836–842. doi: 10.1046/j.1365-2826.2002.00853.x. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Zhu YS, Daniel S, Koibuchi N, Chin WW, Pfaff D. Crosstalk between oestrogen receptors and thyroid hormone receptor isoforms results in differential regulation of the preproenkephalin gene. Journal of Neuroendocrinology. 2001;13:779–790. doi: 10.1046/j.1365-2826.2001.00693.x. [DOI] [PubMed] [Google Scholar]
- Venero C, Guadano-Ferraz A, Herrero AI, Nordstrom K, Manzano J, de Escobar GM, Bernal J, Vennstrom B. Anxiety, memory impairment, and locomotor dysfunction caused by a mutant thyroid hormone receptor alpha1 can be ameliorated by T3 treatment. Genes & Development. 2005;19:2152–2163. doi: 10.1101/gad.346105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis K, Sjogren M, van Hogerlinden M, Silberberg G, Fisahn A, Nordstrom K, Larsson L, Westerblad H, Morreale de Escobar G, Shupliakov O, Vennstrom B. Locomotor deficiencies and aberrant development of subtype-specific GABAergic interneurons caused by an unliganded thyroid hormone receptor alpha1. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:1904–1915. doi: 10.1523/JNEUROSCI.5163-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasco EC, Martinez E, Grant KS, St Germain EA, St Germain DL, Galton VA. Determinants of iodothyronine deiodinase activities in rodent uterus.[see comment] Endocrinology. 2003;144:4253–4261. doi: 10.1210/en.2003-0490. [DOI] [PubMed] [Google Scholar]
- Yu D, Zhou H, Yang Y, Jiang Y, Wang T, Lv L, Zhou Q, Yang Y, Dong X, He J, Huang X, Chen J, Wu K, Xu L, Mao R. The bidirectional effects of hypothyroidism and hyperthyroidism on anxiety- and depression-like behaviors in rats. Hormones and behavior. 2015;69:106–115. doi: 10.1016/j.yhbeh.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Zeng H, Schimpf BA, Rohde AD, Pavlova MN, Gragerov A, Bergmann JE. Thyrotropin-releasing hormone receptor 1-deficient mice display increased depression and anxiety-like behavior. Mol Endocrinol. 2007;21:2795–2804. doi: 10.1210/me.2007-0048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.