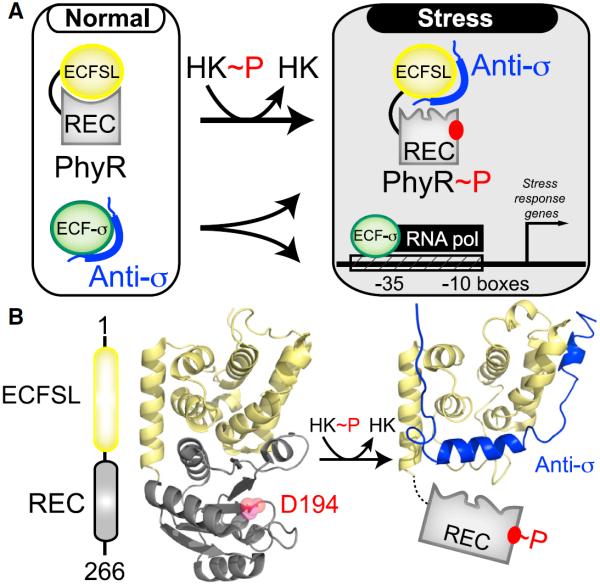
Figure 1. PhyR Regulates General Stress Response in Bacteria by the Regulated “Partner Switching” of an Anti-σ Factor.
(A) Overview of PhyR-based partner switching. Under standard conditions, PhyR adopts a closed conformation with the ECFSL and REC domains, burying surfaces of both domains. Upon phosphorylation by an HK, PhyR changes conformation to expose a binding surface for an anti-σ factor, titrating it away from ECF sigma factors (ECF-σ) and allowing it to enhance the transcription of target genes.
(B) An EL_PhyR domain architecture and homology model, showing how the ECFSL and REC domains bind each other in the unphosphorylated state while not directly occluding the D194 site of phosphorylation. In comparison, a structural model of ECFSL bound to anti-σ suggests that phospho-induced conformational changes will undock the REC and output domains, exposing regions required for anti-σ factor binding (Campagne et al., 2012; Herrou et al., 2012). See also Figure S1.