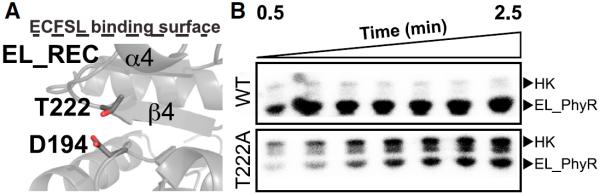
Figure 5. The Isolated REC Domain Displays a Reordered Active Site.
Removal of the output domain leads the α4 helix to adopt a new position, causing reorientation of the conserved residue T222 side chain.
(A) Structure of EL_REC showing that T222 may form a hydrogen bond with the phosphoacceptor D194 mimicking events that would likely happen upon protein phosphorylation.
(B) Phosphotransfer assay showing incorporation of radiolabeled 32P by wild-type (WT) and mutant (T222A) versions of full-length EL_PhyR using HK EL368 (Correa et al., 2013) as a substrate. Reduced incorporation of phosphate by the mutant indicates that residue T222 plays an important role at the phosphotransfer reaction. The marked HK phosphorylation band due to T222A mutation at RR is a result of the reduced phosphotransfer reaction. As the T222A mutation reduces dramatically the intake of phosphate by EL_PhyR, the HK kinase activity rate overcomes the phosphotransfer rate reaction by EL_PhyR. This leads to an increase in the amount of phospho-HK, especially at the beginning of the phosphorylation assay. See also Figures S3, S4, and S7.