Abstract
Background
Mesenteric tumor deposits (MTDs) are not included in the American Joint Committee on Cancer (AJCC) staging system for midgut small intestinal neuroendocrine tumors (NETs). We examined the prognostic significance of MTDs associated with midgut NETs.
Materials and Methods
H&E slides from 132 resected jejunal/ileal NETs were reviewed for AJCC tumor stage, lymph node (LN) metastasis, MTDs, and hepatic metastases. MTDs were defined as discrete irregular mesenteric tumor nodules discontinuous from the primary tumor. Clinical or pathological evidence of metastases and survival data were abstracted from electronic medical records.
Results
The cohort included 72 males and 60 females with a median age of 60 years. LN metastasis, MTDs, and liver metastasis were present in 80%, 68%, and 58% of patients, respectively. Female sex and presence of MTDs were independent predictors of liver metastasis. The odds ratio for hepatic metastasis in the presence of MTDs was 16.68 (95% CI, 4.66–59.73) and 0.81 (95% CI, 0.20–3.26) for LN metastasis. Age, MTDs, and hepatic metastasis were associated with disease-specific survival (DSS) in univariate analysis. Primary tumor histologic grade, pT3/T4 stage and LN metastasis were not associated with DSS. Multivariate analysis of liver metastasis-free survival stratified by tumor grade showed that MTDs were associated with adverse outcomes. The hazard ratio for MTDs was 4.58 (95% CI, 1.89 – 11.11), compared to 0.98 (95% CI, 0.47 – 2.05) for LN metastasis.
Conclusion
MTDs, but not LN metastasis, in midgut neuroendocrine tumors are a strong predictor for hepatic metastasis and are associated with poor DSS.
INTRODUCTION
Gastrointestinal (GI) neuroendocrine tumors (NETs) are typically low grade malignancies that arise from the diffuse neuroendocrine system scattered throughout the gut mucosa.1,2 Midgut NETs (arising in the jejunum and ileum) are among the most common NETs of the GI tract1,3–10. Most primary midgut NETs are small and asymptomatic. However, distant metastasis is present at the time of diagnosis in approximately half of all patients.5,6,9,11 The liver is the most commonly involved site of metastasis11–13, and most patients with midgut NETs die from extensive hepatic disease and liver failure.
Potential prognostic factors for midgut NETs include histologic grade12,14,15 and tumor stage, including lymph node (LN) and distant metastasis9,11,15. However, our previous study has demonstrated that LN metastasis may not necessarily predict poor prognosis in patients with midgut NETs.13 Extramural tumor deposits in the pericolorectal soft tissues or mesentery is a well-recognized adverse prognostic factor for colorectal cancers (CRCs).16–18 Similar tumor deposits, referred to here as mesenteric tumor deposits (MTDs), are a frequent finding in midgut NETs. MTDs in midgut NETs are defined as discrete mesenteric tumor nodules with irregular contours, which are frequently located adjacent to neurovascular bundles and have no associated lymphoid tissue. We have previously shown that, similar to CRC extramural tumor deposits, MTDs may be an important prognostic factor for midgut NETs13. However, reporting the presence of MTDs is not included in the College of American Pathologists Protocol or considered in the current American Joint Committee on Cancer (AJCC) staging system (7th ed.) for midgut NETs18.
In this study, we included 132 patients with resected midgut NET(s), and were able to delineate the prognostic role of LN metastasis and MTDs in these patients. We demonstrate that MTDs associated with midgut NETs are a stronger indicator than LN metastases for liver metastasis and overall prognosis.
MATERIALS AND METHODS
The Surgical Pathology archives at Vanderbilt University Medical Center were searched for midgut small intestine (jejunal/ileal) NETs resected between 1990 and 2015. Cases were excluded if hematoxylin and eosin (H&E)-stained slides were unavailable for review or clinical follow-up data was unobtainable.
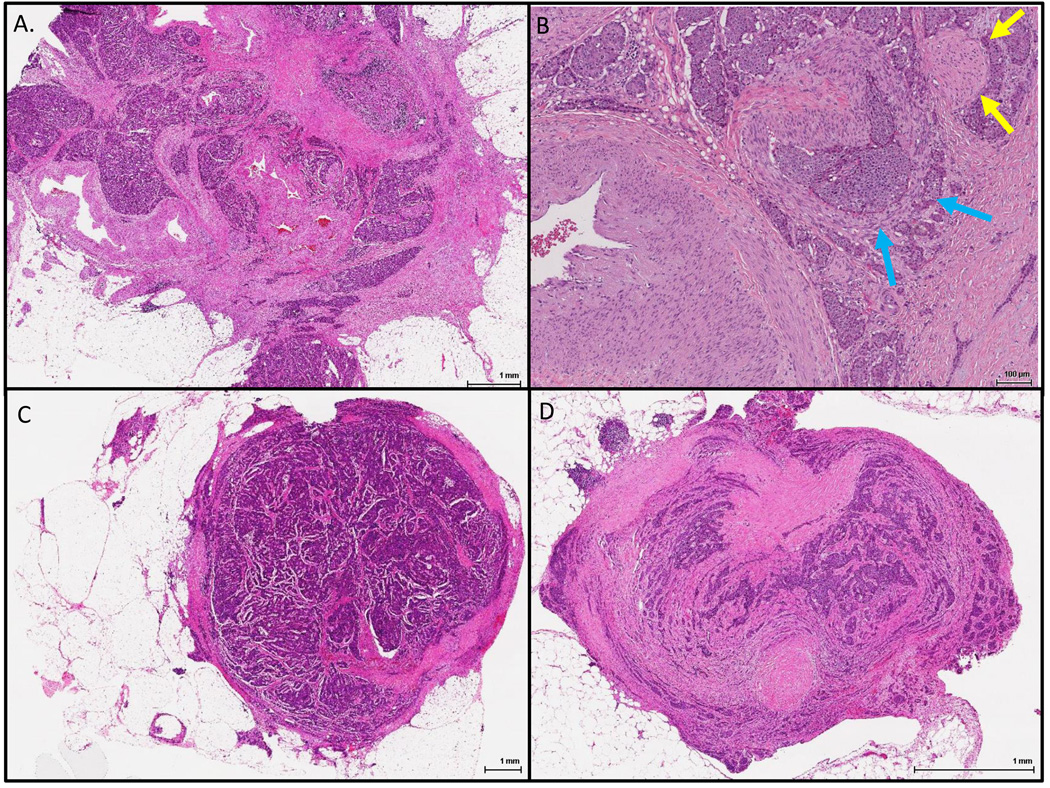
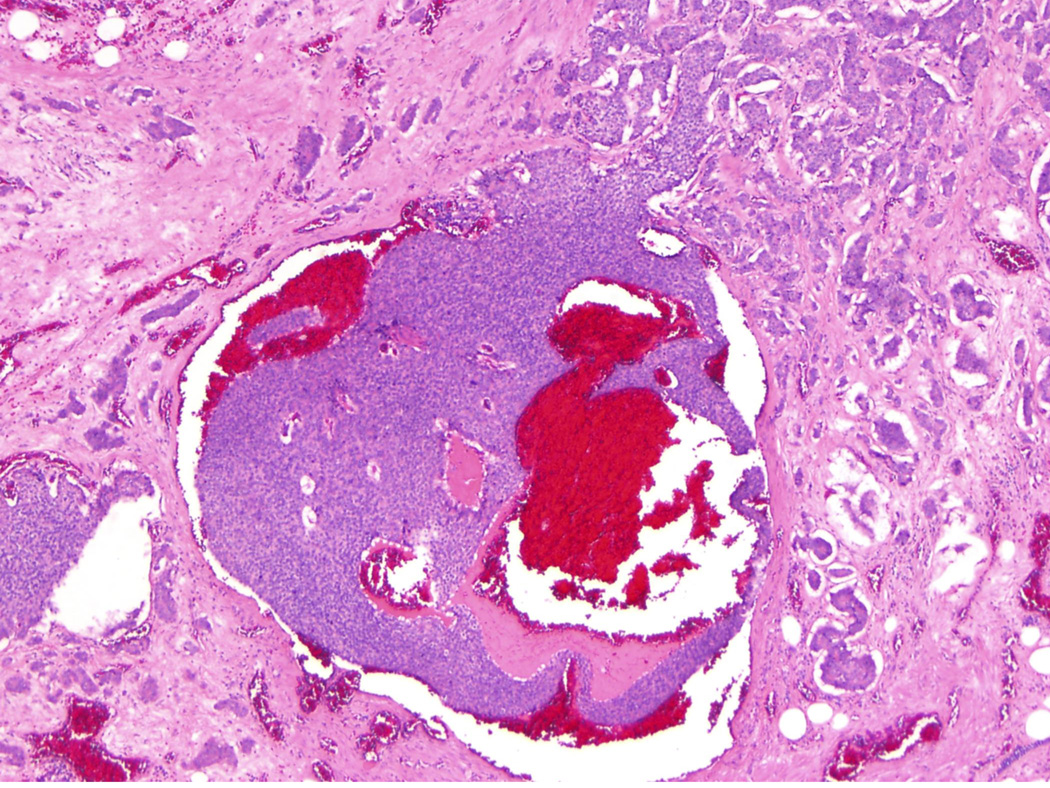
One hundred and thirty two cases were identified, with or without liver resection. Three gastrointestinal pathologists (CRF, RSG and CS) reviewed H&E-stained slides and recorded the AJCC primary tumor stage (pT) and presence or absence of LN (pN) and liver metastasis (pM); patients were staged according to the 7th Ed. of the AJCC Cancer Staging Manual.18 In addition, the presence or absence of MTDs was recorded. MTDs (Figure 1A and 1B) were defined as discrete but irregular mesenteric tumor nodules frequently located adjacent to neurovascular bundles and discontinuous from the primary neoplasm; direct mesenteric extension from the primary tumor or extranodal extension of an involved lymph node was not considered as an MTD. Mesenteric deposits with a rounded contour or associated with a surrounding rim of lymphocytes were considered as LN metastases (Figure 1C), and not MTDs. In addition, tumor nodules involving the peritoneum without adjacent neurovascular bundles were considered as distant peritoneal deposits (Figure 1D). Partial occlusion of large veins by tumor can be seen in some MTDs (Figure 2)
Figure 1.
Representative mesenteric tumor deposits. A. A mesenteric tumor deposit with an irregular contour and associated fibrosis encasing large vessels; B. A higher power view of another mesenteric tumor deposit showing a possible vein involved by tumor (blue arrows) and an entrapped nerve; C. A lymph node completely replaced by neuroendocrine tumor with extra-nodal extension; D. A peritoneal tumor implant with partial mesothelial lining and containing no large vessels.
Figure 2.
A mesenteric tumor deposit with a vein containing a large tumor cluster (original magnification 40X).
Electronic medical records were reviewed for patient demographics and follow-up data, including disease-specific survival. The presence of distant metastasis was documented by review of radiology and pathology reports. The Ki67 proliferative index of the primary tumors was available for 110 of 132 cases. A representative formalin-fixed paraffin-embedded tumor section from each primary tumor was used for immunohistochemical labeling for Ki67 (Dako, Carpinteria, CA; dilution 1:100). Ki67 proliferative index was calculated as a percentage of 500–2,000 tumor cells that stained positive in the areas of highest nuclear labeling. This information was used to grade midgut NETs according to 2010 World Health Organization (WHO) criteria.19 The study protocol was approved by the Institutional Review Board at Vanderbilt University.
Associations between MTDs and other clinicopathologic variables with hepatic metastasis were assessed using univariate and multivariate logistic regression. Disease-specific survival was assessed using Cox proportional hazard regression. For multivariate analysis of metastasis-free survival analysis stratified by tumor grade, patients with liver metastasis at the initial diagnosis were set to 1 day metastasis-free survival. All hypothesis tests were two-sided with α=0.05. All statistical analyses were performed using Stata v13.1 (Stata Corporation, College Station, TX).
RESULTS
Demographic and Clinicopathologic Features
Demographics and clinicopathologic features are summarized in Table 1. Sixty-eight of 132 (51.5%) patients had liver metastases at initial diagnosis, and 9 other patients (6.8%) developed liver metastases during clinical follow-up. Among these 77 cases with liver metastasis, 37 (48.1%) were described as “numerous liver lesions” in radiology reports. Median follow-up time was 48 months (range, 1–190 months). Ninety-six patients were alive at last clinical follow-up, 25 died of disease, and 11 died of other causes. Among the 96 surviving patients, 59 (61.6%) had evidence of residual or recurrent disease, primarily manifesting as hepatic metastases. The average number of lymph nodes sampled in each case was 12 (SD=10; range 0–46).
Table 1.
Overall Patient Demographics and Clinicopathologic Features
| Total cases (N=132) | ||
|---|---|---|
| Age (years) | 60 (19–85) | |
| Sex | Female | 60 (45.4%) |
| Male | 72 (55.5%) | |
| T stage | 1 | 6 (4.5%) |
| 2 | 15 (11.4%) | |
| 3 | 67 (50.8%) | |
| 4 | 39 (29.5%) | |
| n/a | 5 (3.8%) | |
| Lymph node metastasis | Absent | 22 (16.7%) |
| Present | 106 (80.3%) | |
| n/a | 4 (3.0%) | |
| Mesenteric tumor deposit | Absent | 41 (31.1%) |
| Present | 86 (65.1%) | |
| n/a | 5 (3.8%) | |
| Liver metastasis | Absent | 55 (41.7%) |
| Present | 77 (58.3%) | |
| WHO grade | Grade 1 | 86 (65.1%) |
| Grade 2 | 22 (16.7%) | |
| n/a | 24 (18.2%) | |
| Follow up (months) | 48 (1–190) | |
| Survival status | Alive | 96 (72.7%) |
| Died of disease | 25 (19.0%) | |
| Died of other causes | 11 (8.3% | |
MTDs, but not LN metastasis, are associated with liver metastasis
Univariate and multivariate logistic regression was performed to determine which factor(s) predicted liver metastasis at presentation and during the follow-up. Only female sex and the presence of MTDs were independent predictors of liver metastasis (Table 2). Other factors including age, advanced pT stage, LN metastasis, and histologic grade were not significantly associated with liver metastasis in patients with midgut NETs. The odd ratios for liver metastasis at presentation were 14. 36 (95% CI, 4.01–51.59; p<0.001) for MTDs and 1.28 (95% CI, 0.29 – 5.67; p=0.75) for LN metastasis.
Table 2.
Potential predictors for Liver metastasis in patients with midgut neuroendocrine tumor
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Female sex | 0.34 | 0.17–0.71 | 0.004* | 0.21 | 0.07–0.61 | 0.004* |
| Age (years) | 3.03 | 0.48–18.92 | 0.392 | 1.00 | 0.95–1.04 | 0.852 |
| pT3 | 2.10 | 0.78–5.68 | 0.143 | 1.26 | 0.27–5.86 | 0.767 |
| pT4 | 1.65 | 0.56–4.83 | 0.363 | 0.57 | 0.10–3.19 | 0.526 |
| LN metastasis | 1.8 | 0.71–4.54 | 0.213 | 0.81 | 0.20–3.26 | 0.767 |
| MTD | 9.58 | 3.97–23.13 | <0.001* | 16.68 | 4.66–59.73 | <0.001* |
| Histologic grade | 1.28 | 0.50–3.32 | 0.770 | 1.51 | 0.46–4.90 | 0.495 |
Abbreviations: OR, odd ratio; CI, confidence interval; LN, lymph node; MTD, mesenteric tumor deposits.
p<0.05.
Note: Age was entered as a continuous variable; pT3 and pT4 tumors were compared to combined pT1 and pT2 tumors; WHO grade 1 was the reference for histologic grade.
MTDs were present in 73% (62/85) of cases with liver metastasis, whereas only 22% (9/41) of cases without MTDs had liver metastasis (Fisher's exact test, p<0.001). Among 37 patients with numerous liver metastases (at least 15 metastatic deposits), 34 (92%) had MTDs, 1 had no MTDs and 2 had no information about MTDs. There were 28 cases with LN metastasis but no MTDs; 8 of these cases (29%) had liver metastasis. Among these 8 cases, 1 (4% of the 28 cases) had numerous liver lesions. Contrariwise, 10 of 11 (91%) patients with MTDs but no LN metastasis had liver metastasis. Among these 10 cases, 6 (55% of the 11 cases) had numerous liver lesions.
Presence of MTDs, but not LN metastasis, is associated with poor prognosis
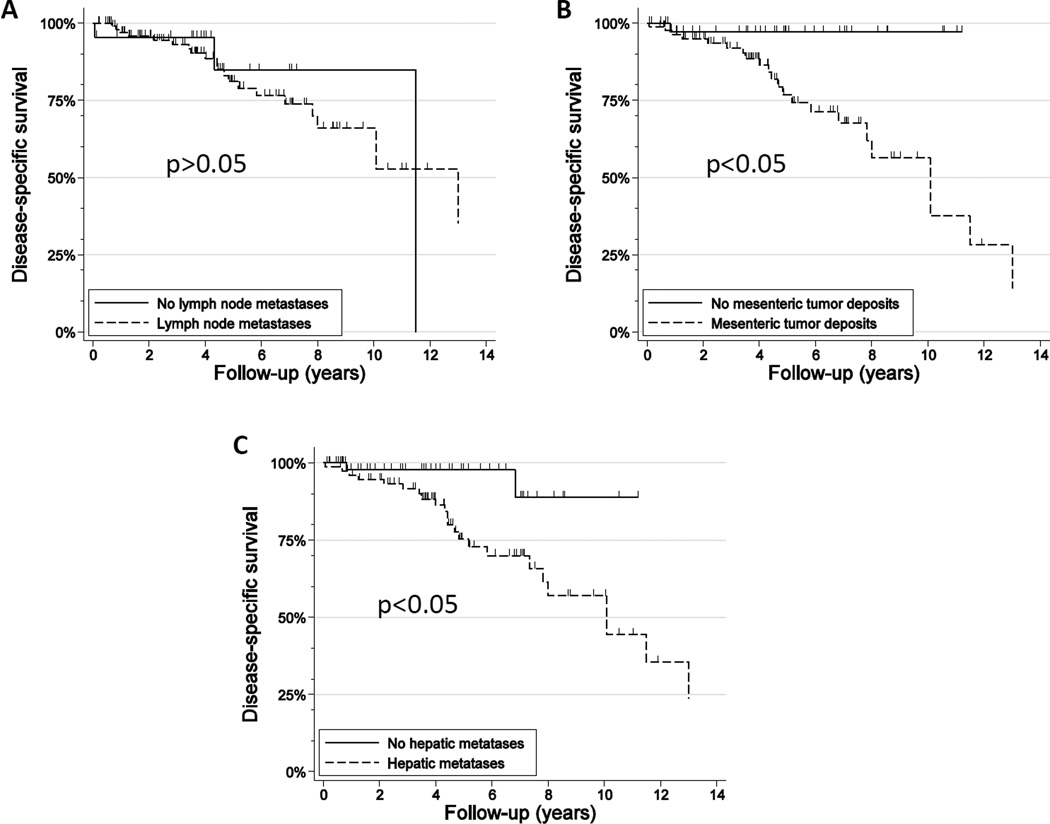
Increasing age, liver metastasis, and MTDs all showed increased hazard ratios for disease-specific survival in univariate Cox proportional-hazards regression (Table 3, Figure 3). Notably, advanced T stage, LN metastasis, and histologic grade of the primary tumors showed no significant effects on disease specific survival.
Table 3.
Potential predictors for disease specific survival in patients with midgut neuroendocrine tumor
| Univariate analysis | MFS (multivariate analysis) | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Sex | 0.96 | 0.43–2.13 | 0.920 | 0.61 | 0.35–1.05 | 0.072 |
| Age | 1.10 | 1.05–1.15 | <0.001* | |||
| pT2 | 1.10 | 0.13–9.49 | 0.930 | |||
| pT3 | 1.52 | 0.33–7.04 | 0.586 | 1.26 | 0.17–9.35 | 0.823 |
| pT4 | 2.80 | 0.60–12.85 | 0.185 | 1.02 | 0.13–7.88 | 0.988 |
| LN metastasis | 1.19 | 0.35–4.05 | 0.775 | 0.98 | 0.47–2.05 | 0.967 |
| MTD | 11.89 | 1.60–88.45 | 0.016* | 4.58 | 1.89–11.11 | 0.001* |
| Liver metastasis | 5.08 | 1.19–21.75 | 0.028* | |||
| Histologic grade | 0.71 | 0.16–3.21 | 0.653 | |||
Abbreviations: MSF, metastasis-free survival; HR, hazard ratio; CI, confidence interval; LN, lymph node; MTD, mesenteric tumor deposit.
p<0.05.
Note: Age was entered as a continuous variable; pT3 and pT4 tumors were compared to combined pT1 and pT2 tumors in the univariate analysis; pT2, pT3, and pT4 tumors were compared to T1 in the multivariate analysis; the multivariate analysis was stratified by grade. WHO grade 1 was the reference category for histologic grade in the univariate analysis.
Figure 3.
Kaplan-Meier survival curves of patients with lymph node metastasis versus those without lymph node metastasis (A), with mesenteric tumor deposits versus those without mesenteric tumor deposits (B), with liver metastasis versus without liver metastasis (C).
Sex, pT stage, LN metastasis, and MTD were included in a multivariate Cox regression model to assess liver metastasis-free survival upon stratification by tumor grade. The presence of MTDs was the only variable associated with adverse outcomes (Table 3). Interestingly, the hazard ratio for MTDs was 4.58 (95% CI, 1.89 − 11.11; p=0.001), compared to 0.98 (95% CI, 0.47 − 2.05; p=0.967) for LN metastasis.
DISCUSSION
LN metastasis has been considered a poor prognostic factor for midgut NETs.18 However, our data showed that LN metastasis was not predictive of liver metastasis and had no association with disease-specific survival. This discrepancy may be due to the common occurrence of lymph node metastasis in our series (80% of patients in this cohort had lymph node metastasis), thereby limiting its discriminatory power as a prognostic factor. Nevertheless, results from a recent large cohort study demonstrating no survival differences between AJCC stage I/II (T1-3N0M0) versus stage III (T4N0M0 or T1-4N1M0) would seem to support our findings.11
MTDs are common in patients with midgut NETs, but their clinical significance has not been thoroughly investigated.13 Our previous study on 72 patients with NETs of the midgut suggested that the presence of MTDs was associated with LN and liver metastasis, as well as an increase in hazard of progression or death due to disease.13 Here, we present further evidence that MTDs are a strong predictor for liver metastases in patients with midgut NET. More importantly, this study demonstrated that MTDs are stronger indicator than LN metastasis for liver metastasis and overall prognosis.
In colorectal adenocarcinoma, discontinuous extramural tumor deposits are thought to be histopathologic manifestations of venous invasion, lymphatic invasion, or nerve sheath infiltration.21 Here, we observed that almost all MTDs were located adjacent to medium or large-sized vessels. In some MTDs, partial occlusion of a large vein by tumor can be seen (Figure 2). In other cases, arteries, without identifiable accompanying veins, are encased by tumor. Therefore, it seems probable that venous invasion is the initial step for development of MTDs. With tumor progression, the involved veins are likely completely obliterated. Entrapped nerves are frequently present in advanced MTDs as well. However, small MTDs usually lack entrapped nerve fibers. Therefore, we consider that MTDs are less likely to result from nerve sheath infiltration. Since MTDs seem to arise from venous invasion, it is reasonable to assume that tumor cells in MTDs have access to the enterohepatic venous system, which then give rise to hepatic metastasis.
Several studies have reported the prognostic significance of histologic grade in midgut NETs. However, most of these studies either did not use current WHO criteria11,15 or graded metastatic deposits instead of the primary tumor.14,20,22 In this study, 110 tumors were graded based on Ki67 proliferative indices of the primary tumor. We found that histologic grade of primary tumors was not associated with liver metastasis or decreased disease specific survival. Previously, we reported that patients with WHO grade 1 midgut NET may develop metastatic liver lesions of any histologic grade, including those with Ki67 labeling indices >20% (WHO grade 3).23 Patients with such liver lesions had shorter progression-free survival compared to those with liver lesions of lower histologic grade. Therefore, the prognostic role of histologic grade in primary midgut NETs should be investigated further in large-scale studies. Additionally, histologic grade of the metastatic lesion(s) should also be assessed in patients with distant spread of disease to better predict disease progression.
In summary, MTDs are a common finding in patients with midgut NET. MTDs are a strong predictor for liver metastasis and as a corollary, decreased disease-specific survival. In contrast, LN metastasis was not significantly associated with liver metastasis or disease specific survival. Therefore, we conclude that the presence or absence of MTDs should be reported for midgut NETs and be considered for inclusion in the next iteration of the AJCC cancer staging algorithm for these tumors. In our opinion, the presence of MTDs should be considered as a more advanced stage than Stage IIIB (T1-4N1), perhaps as Stage IIIC.
Acknowledgments
Grant Support
NIH/NIDDK 5P30 DK058404-13 (CS)
NIH/NCI 5P50 CA095103-13 (CS)
Footnotes
Disclosures: The authors have no conflicts of interest
REFERENCES
- 1.Mocellin S, Nitti D. Gastrointestinal carcinoid: epidemiological and survival evidence from a large population-based study (n = 25 531) Ann Oncol. 2013;24:3040–3044. doi: 10.1093/annonc/mdt377. [DOI] [PubMed] [Google Scholar]
- 2.Solcia E, Vanoli A. Histogenesis and natural history of gut neuroendocrine tumors: present status. Endocr Pathol. 2014;25:165–170. doi: 10.1007/s12022-014-9312-0. [DOI] [PubMed] [Google Scholar]
- 3.Haselkorn T, Whittemore AS, Lilienfeld DE. Incidence of small bowel cancer in the United States and worldwide: geographic, temporal, and racial differences. Cancer Causes Control. 2005;16:781–787. doi: 10.1007/s10552-005-3635-6. [DOI] [PubMed] [Google Scholar]
- 4.Hauso O, Gustafsson BI, Kidd M, et al. Neuroendocrine tumor epidemiology: contrasting Norway and North America. Cancer. 2008;113:2655–2664. doi: 10.1002/cncr.23883. [DOI] [PubMed] [Google Scholar]
- 5.Modlin IM, Champaneria MC, Chan AK, et al. A three-decade analysis of 3,911 small intestinal neuroendocrine tumors: the rapid pace of no progress. Am J Gastroenterol. 2007;102:1464–1473. doi: 10.1111/j.1572-0241.2007.01185.x. [DOI] [PubMed] [Google Scholar]
- 6.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence B, Gustafsson BI, Chan A, et al. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:1–18. vii. doi: 10.1016/j.ecl.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Tsikitis VL, Wertheim BC, Guerrero MA. Trends of incidence and survival of gastrointestinal neuroendocrine tumors in the United States: a seer analysis. J Cancer. 2012;3:292–302. doi: 10.7150/jca.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 10.Fraenkel M, Kim MK, Faggiano A, et al. Epidemiology of gastroenteropancreatic neuroendocrine tumours. Best Pract Res Clin Gastroenterol. 2012;26:691–703. doi: 10.1016/j.bpg.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Strosberg JR, Weber JM, Feldman M, et al. Prognostic validity of the American Joint Committee on Cancer staging classification for midgut neuroendocrine tumors. J Clin Oncol. 2013;31:420–425. doi: 10.1200/JCO.2012.44.5924. [DOI] [PubMed] [Google Scholar]
- 12.Panzuto F, Campana D, Fazio N, et al. Risk factors for disease progression in advanced jejunoileal neuroendocrine tumors. Neuroendocrinology. 2012;96:32–40. doi: 10.1159/000334038. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez RS, Liu EH, Alvarez JR, et al. Should mesenteric tumor deposits be included in staging of well-differentiated small intestine neuroendocrine tumors? Mod Pathol. 2014;27:1288–1295. doi: 10.1038/modpathol.2013.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhall D, Mertens R, Bresee C, et al. Ki-67 proliferative index predicts progression-free survival of patients with well-differentiated ileal neuroendocrine tumors. Hum Pathol. 2012;43:489–495. doi: 10.1016/j.humpath.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Palazzo M, Lombard-Bohas C, Cadiot G, et al. Ki67 proliferation index, hepatic tumor load, and pretreatment tumor growth predict the antitumoral efficacy of lanreotide in patients with malignant digestive neuroendocrine tumors. Eur J Gastroenterol Hepatol. 2013;25:232–238. doi: 10.1097/MEG.0b013e328359d1a6. [DOI] [PubMed] [Google Scholar]
- 16.Lo DS, Pollett A, Siu LL, et al. Prognostic significance of mesenteric tumor nodules in patients with stage III colorectal cancer. Cancer. 2008;112:50–54. doi: 10.1002/cncr.23136. [DOI] [PubMed] [Google Scholar]
- 17.Puppa G, Maisonneuve P, Sonzogni A, et al. Pathological assessment of pericolonic tumor deposits in advanced colonic carcinoma: relevance to prognosis and tumor staging. Mod Pathol. 2007;20:843–855. doi: 10.1038/modpathol.3800791. [DOI] [PubMed] [Google Scholar]
- 18.Edge SB, Fritz AG, Byrd DR, et al., editors. AJCC Cancer Staging Manual. 7th. Vol. 151. New York: Springer; 2009. pp. 181–185. [Google Scholar]
- 19.Bosman FT, Carneiro F, Hruban RH, editors. WHO Classification of Tumours of the Digestive System. 4th. Lyon: International Agency for Research on Cancer (IARC); 2010. [Google Scholar]
- 20.Ahmed A, Turner G, King B, et al. Midgut neuroendocrine tumours with liver metastases: results of the UKINETS study. Endocr Relat Cancer. 2009;16:885–894. doi: 10.1677/ERC-09-0042. [DOI] [PubMed] [Google Scholar]
- 21.Wunsch K, Muller J, Jahnig H, et al. Shape is not associated with the origin of pericolonic tumor deposits. Am J Clin Pathol. 2010;133:388–394. doi: 10.1309/AJCPAWOLX7ADZQ2K. [DOI] [PubMed] [Google Scholar]
- 22.Strosberg J, Nasir A, Coppola D, et al. Correlation between grade and prognosis in metastatic gastroenteropancreatic neuroendocrine tumors. Hum Pathol. 2009;40:1262–1268. doi: 10.1016/j.humpath.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Shi C, Gonzalez RS, Zhao Z, et al. Liver metastases of small intestine neuroendocrine tumors: Ki-67 heterogeneity and World Health Organization grade discordance with primary tumors. Am J Clin Pathol. 2015;143:398–404. doi: 10.1309/AJCPQ55SKOCYFZHN. [DOI] [PMC free article] [PubMed] [Google Scholar]