Abstract
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal of malignancies, in large measure, due to the propensity of PDAC cells to acquire resistance to chemotherapeutic agents. A better understanding of the molecular basis of acquired resistance is a major focus of contemporary PDAC research. We report here the results of a study to independently develop cisplatin resistance in two distinct parental PDAC cell lines, AsPC1 and BxPC3, and to subsequently examine the molecular mechanisms associated with the acquired resistance. Cisplatin resistance in both resistant cell lines was found to be multifactorial and to be associated with mechanisms related to drug transport, drug inactivation, DNA damage response, DNA repair and the modulation of apoptosis. Our results demonstrate that the two resistant cell lines employed alternative molecular strategies in acquiring resistance dictated, in part, by pre-existing molecular differences between the parental cell lines. Collectively, our findings indicate that strategies to inhibit or reverse acquired resistance of PDAC cells to cisplatin, and perhaps other chemotherapeutic agents, may not be generalized but will require individual molecular profiling and analysis to be effective.
Introduction
Pancreatic ductal adenocarcinoma (PDAC), the most common type of pancreatic cancer,1 is among the most lethal of malignancies, with an estimated 5-year survival rate in the United States of only 7.2%.2 Major reasons for this poor prognosis include the following: (i) late diagnosis with about two-thirds of patients presenting locally advanced or metastatic disease, for which curative surgery is not available;3 (ii) aggressive clinical behavior with rapid progression through local and distant metastases; and (iii) intrinsic resistance to conventional chemotherapy and radiotherapy.4 In addition, even if early stages of PDAC are treated by surgical resection with curative intent, recurrent or metastatic disease can develop in long-term survivors.5 As a result, effective systemic therapy (chemotherapy and/or immunotherapy) is clearly needed to better control this biologically aggressive disease.
For the last two decades, standard first-line treatment for locally advanced and metastatic PDAC relied on gemcitabine and more recently on its combination with the targeted agent erlotinib or the albumin-bound cytotoxic agent paclitaxel.6 Another combination of four drugs, that is, the platinum agent oxaliplatin together with irinotecan, fluorouracil and leucovorin (Folfirinox), has shown modest improvement in response rates, overall survival and progression-free survival over treatment with single-agent gemcitabine.7
Another platinum agent, cisplatin, is also being evaluated as a prospective addition to the combined chemotherapy of early, advanced or metastatic PDAC in several ongoing clinical trials (for example, trials NCT01150630 and NCT01593475, https://clinicaltrials.gov/). The addition of cisplatin to gemcitabine or other established drugs for the treatment of PDAC is reasonable, as cellular response to cisplatin is regulated by the Fanconi anemia/BRCA pathway8 that has been shown to be disrupted in a number of pancreatic cancers.9, 10 Thus, pancreatic cancer cells may reasonably be expected to be sensitive to cisplatin.
Cisplatin displays a broad spectrum of anticancer activity, and is estimated to be administered to 40–80% of all cancer patients undergoing chemotherapy;11 however, its clinical utility is often limited due to acquired drug resistance and adverse side effects.12, 13 Consequently, understanding of the mechanisms involved in the resistance of PDAC cells to cisplatin is highly desirable as this insight may help to refine the use of cisplatin in pancreatic cancer chemotherapy. The purpose of this study was to independently develop two cisplatin-resistant pancreatic cancer cell lines from different parental PDAC cell lines and to subsequently examine the molecular mechanisms associated with their acquired resistance.
Materials and methods
Reagents and assay kits
Cisplatin (Product No. P4394) and TOX8 In Vitro Toxicology Assay Kit were purchased from Sigma-Aldrich (St. Louis, MO, USA). A stock solution of cisplatin was prepared at a concentration of 0.5 mg ml–1 in 0.9% NaCl and stored in the dark at 4 °C.
Cell cultures and treatments
The human PDAC cell lines AsPC1 (CRL-1682)14 and BxPC3 (CRL-1687)15 were obtained from ATCC (Manassas, VA, USA) and maintained in RPMI 1640 with L-glutamine (Mediatech, Inc., Manassas, VA, USA) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA, USA) and 1% antibiotic-antimycotic solution (Mediatech, Inc.). The PDAC cell lines AsPC1-R and BxPC3-R resistant against cisplatin were developed from the respective low-passage number parental cell lines AsPC114 and BxPC3,15 by culturing in medium with step-wise increasing concentrations of cisplatin as previously described.16 Parental cells were seeded into tissue culture-treated flasks in full RPMI 1640 medium with 10% fetal bovine serum, 2 mM L-glutamine, penicillin (100 IU ml–1), streptomycin (100 μg ml–1) and amphotericin B (0.25 μg ml–1), and cisplatin was added 24 h later when cell density was around 20% at a concentration equal to IC20. As the cultures became confluent, the cells were sub-cultured and cisplatin was added to the medium with step-wise increases of concentration. Response of parental and resistant cell lines to cisplatin was determined by the resazurin-based (TOX8) cell viability assay following 72-h treatment with different concentrations of cisplatin. IC50 values were determined as previously described and expressed as average±s.e.17
Gene expression analysis by DNA microarrays
Gene expression profiling of parental and resistant cell lines was performed using GeneChip Gene 1.0 ST Arrays (Affymetrix, Santa Clara, CA, USA). Total cellular RNA was isolated from exponentially growing AsPC1, AsPC1-R, BxPC3 and BxPC3-R cells in drug-free media. Fragmented and biotin-labeled cDNAs were prepared and hybridized onto arrays (three arrays per cell type). Hybridization data were processed and normalized by robust multi-array average-sketch and submitted to the Gene Expression Omnibus repository and are publicly available under the series accession number GSE73978.
Significantly differentially expressed genes for both resistant/sensitive cell pairs were identified using Significance Analysis of Microarrays.18 Fold change for genes upregulated in resistant cells was expressed as a ratio of their normalized signal intensities in resistant vs parental cells; fold change for genes downregulated in resistant cells was expressed as a negative ratio of their normalized signal intensities in parental vs resistant cells. Genes with absolute fold change ⩾1.5 and false discovery rate (FDR) 0% were considered to be significantly differentially expressed. Differential expression of selected genes was validated by quantative PCR gene expression assays (for details see Supplementary Method and Results). Genes whose changes in expression had been previously associated with cisplatin resistance in cancer cells were identified from previously published reports,19, 20, 21, 22, 23, 24 and a 72-member list of cisplatin resistance-related genes was created (Supplementary Material, Supplementary Table S1). Cisplatin resistance-related genes that were differentially expressed between at least one pair of resistant/parental cell lines were also examined for differential expression between parental AsPC1 and BxPC3 cells to assess the possible contribution of their baseline expression differences to the differences in their expression between resistant/sensitive pairs.
Functional analysis of gene expression data
Probesets corresponding to differentially expressed genes were used in the pathway enrichment analysis using the MetaCore suite 6.23 build 67496 (Thomson Reuters, New York, NY, USA) that identifies significantly perturbed pathways by mapping differentially expressed genes onto manually curated pathway maps.25 Pathways were considered to be significantly enriched if their q-values were⩽FDR threshold, for which the expected number of false-positive entities was⩽1.
Gene sets significantly enriched in resistant and sensitive phenotypes were identified by the Gene Set Enrichment Analysis (GSEA) method26 using robust multi-array average-sketch processed data without filtering of probesets, using categorical phenotype labels, gene set permutation type and signal-to-noise metrics. Gene sets included in the analysis were the following: H: Hallmark (50 gene sets) and C6: Oncogenic Signatures (189 gene sets) from the Molecular Signatures Database (URL: http://www.broadinstitute.org/gsea/msigdb/collections.jsp). The results of GSEA were visualized by the network-based ‘Enrichment Map' method27 implemented as Enrichment Map plugin v 2.0.1 in Cytoscape v 3.2.1[ref. 28]. In the pathway enrichment analyses, the statistical significance of enrichment was evaluated using calculated P-values based on the hypergeometric distribution and corrected for multiplicity using the FDR procedure. The Enrichment Map parameters used were as follows: P-value cutoff: 0.001; FDR q-value cutoff: 0.05; Similarity cutoff (overlap coefficient): 0.5; and combined constant: 0.5.
Statistics
Unless stated otherwise, statistical analysis of the data was performed using GraphPad Prism 5.02 for Windows (GraphPad Software, San Diego, California USA), and differences were considered significant for two-sided P-value<0.05.
Results
Resistant pancreatic cell lines were established by step-wise increase in concentrations of cisplatin over more than 20 serial passages
The pancreatic cancer cell lines, AsPC1 and BxPC3, which display considerable genotypic and phenotypic differences from one another (see Supplementary Material, Supplementary Table S2), were used for development of resistance to cisplatin through exposure to step-wise increases in concentrations of the drug over more than 20 passages. The resulting cell lines, AsPC1-R and BxPC3-R, are both significantly more resistant to the drug than their respective parental cells (AsPC1 IC50=20.07±1.22 μm, AsPC1-R IC50=29.44±1.75 μM, P<0.05, t-test; BxPC3 IC50=0.85±1.12 μM, BxPC3-R IC50=12.63±1.19 μM, P<0.05, t-test). Although AsPC1-R cells acquired a higher absolute resistance to cisplatin than BxPC3-R cells, they displayed a lower fold increase in resistance than BxPC3-R cells relative to their respective parental cell lines (1.5 × vs 14.9 × ). The resistant cell lines displayed the same morphology as their respective parental cell lines (Supplementary Material, Supplementary Figure S1).
Cisplatin-resistant cells display significant differences in gene expression profiles and associated changes in biological pathways and processes
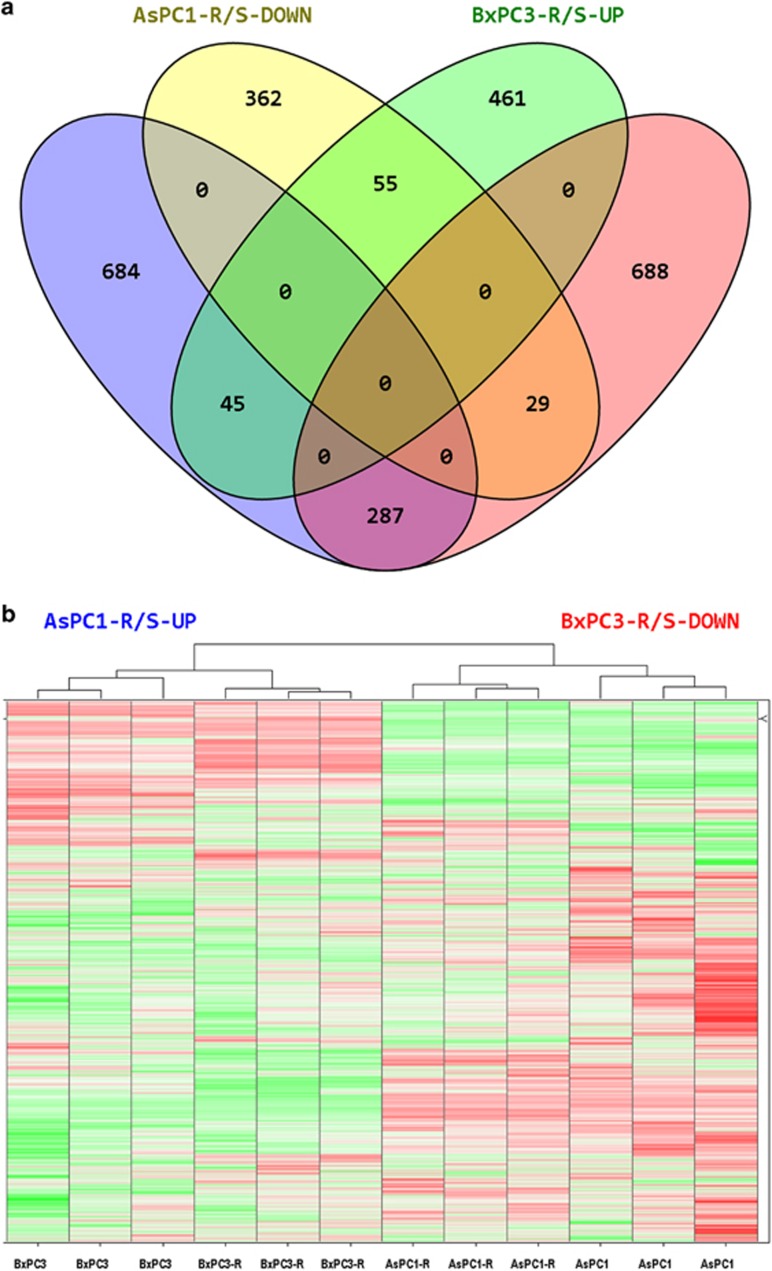
Gene expression analysis identified 1462 differentially expressed genes between AsPC1-R and AsPC1 cells, and 1565 differentially expressed genes between BxPC3-R and BxPC3 cells. Of the 416 genes identified as differentially expressed for both resistant/parental cell pairs, 342 genes (82%) displayed discordant changes in gene expression (Figure 1a). Hierarchical clustering analysis of the gene expression data (33 297 probeset values for 12 specimens) demonstrated that each cisplatin-resistant cell line is more similar to its respective parental cell line than to the other cisplatin-resistant cell line (Figure 1b). This indicates that cisplatin-resistant cell lines, though developed from the same cancer type, can be significantly different in their global gene expression profiles. This difference reflects dissimilarities in the expression profiles of their parental cell lines and/or differences associated with their respective acquisition of cisplatin resistance.
Figure 1.
(a) Number of genes differentially expressed between AsPC1-R vs AsPC1 (AsPC1-R/S) and BxPC3-R vs BxPC3 (BxPC3-R/S) resistant/parental cell pairs. DOWN, downregulated genes; UP, upregulated genes. (b) Hierarchical cluster analysis of gene expression data (33 297 probeset values for 12 specimens); Z-score normalized and green-to-red: Z-score low-to-high.
To evaluate changes in gene expression associated with the acquisition of cisplatin resistance in the two cell lines, we first performed Pathway Enrichment Analysis for genes upregulated and downregulated in each resistant cell line relative to their corresponding parental cell lines. The results indicate that genes upregulated in each of the resistant cell lines were significantly enriched in different pathway maps (Supplementary Material, Supplementary Tables S3–S6). For example, genes upregulated in AsPC1-R cells were enriched in several complement and cytokine-associated immune response pathways and NF-kB signal transduction pathways. In contrast, genes upregulated in BxPC3-R cells were significantly enriched in the cyclic AMP-dependent and Thromboxane A2 signaling pathways and not in complement or cytokine-mediated pathways.
Interestingly, several pathways enriched for genes upregulated in BxPC3-R cells were enriched for genes downregulated in AsPC1-R cells and vice versa (Table 1). For example, the immune response IL-1 Signaling Pathway that was found to be significantly enriched for genes downregulated in BxPC3-R cells (Supplementary Material, Supplementary Table S6) was also found as significantly enriched for genes upregulated in AsPC1-R cells (Supplementary Material, Supplementary Table S3).
Table 1. MetaCore pathway maps identified as significantly enriched by genes upregulated or downregulated in both pairs of cisplatin-resistant/parental cell lines.
| MetaCore pathwaya |
Status of genes enriched in MetaCore pathway |
|
|---|---|---|
| AsPC1-R vs AsPC1 | BxPC3-R vs BxPC3 | |
| Immune response_Alternative complement pathway | Upregulated | Downregulated |
| Immune response_IL-1 signaling pathway | Upregulated | Downregulated |
| Signal transduction_NF-kB activation pathways | Upregulated | Downregulated |
| Immune response_TLR5, TLR7, TLR8 and TLR9 signaling pathways | Upregulated | Downregulated |
| Immune response_HSP60 and HSP70/ TLR signaling pathway | Upregulated | Downregulated |
| Immune response_MIF-induced cell adhesion, migration and angiogenesis | Upregulated | Downregulated |
| Immune response_IL-18 signaling | Upregulated | Downregulated |
| Immune response_IL-33 signaling pathway | Upregulated | Downregulated |
| NETosis in SLE | Downregulated | Upregulated |
| Development_Role of IL-8 in angiogenesis | Downregulated | Downregulated |
Maps are available at Thomson Reuters website URL: http://lsresearch.thomsonreuters.com/maps/.
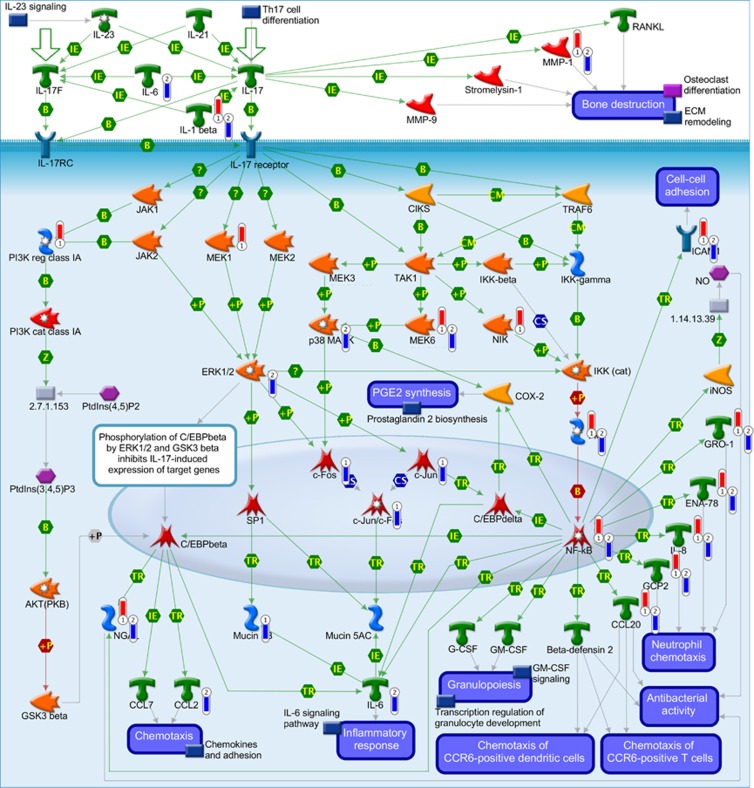
The immune response IL-17 Signaling Pathway was found to be significantly enriched for genes downregulated in BxPC3-R cells (Supplementary Material, Supplementary Table S6) but not for AsPC1-R (FDR, NS). However, when both upregulated and downregulated genes are considered together, the pathway was found to be significantly enriched for both resistant/parental pairs (Figure 2). Among the 49 genes involved in this pathway, 17 were differentially expressed between AsPC1-R/AsPC1 cells and 15 were differentially expressed between BxPC3-R/ BxPC3 cells (for both FDR<0.05). Overlaying the expression data on this pathway map demonstrates that differences in NF-kB activity between the two resistant PDAC cell lines (Table 1) are associated with enhanced activity in AsPC1-R cells and reduced activity in BxPC3-R cells relative to their parental cell lines. This difference in NF-kB activity between the two resistant PDAC cell lines is supported by consistent upregulation of NF-kB transcriptional targets in AsPC1-R cells and their downregulation in BxPC3-R cells relative to their parental counterparts (Figure 2).
Figure 2.
MetaCore Immune response_IL-17 signaling pathway. 1=AsPC1-R vs AsPC1 cells; 2=BxPC3-R vs BxPC3 cells; red thermometer: gene overexpressed in resistant cells; blue thermometer: gene underexpressed in resistant cells. Green arrows indicate activation, red arrows inhibition. The nodes in the graph represent GeneGo Network objects that depict genes and/or gene complexes. For detailed definition of all symbols, see https://portal.genego.com/legends/MetaCoreQuickReferenceGuide.pdf.
The observed differences in NF-kB activity between the resistant cell lines may be additionally understood by examining the MetaCore Map ‘Development_NOTCH1_mediated pathway for NF-KB activity modulation' (Supplementary Figure S2). Overlaying differentially expressed genes for both resistant/parental cell pairs onto this map indicates that differential expression of interleukin 1 (IL-1 alpha), the interleukin 1 receptor (IL-TRI) and the associated kinases IRAK1/2 and MEKK1 may contribute to the observed difference in NF-kB activity (see Supplementary Material, Supplementary Figure S2 and Supplementary Table S3).
The results of the GSEA indicate that the cisplatin-resistant phenotypes for both PDAC cell lines are enriched for different gene sets. In the AsPC1-R/AsPC1 cell pair, the resistant cells are enriched for gene sets relevant to interferon response, inflammatory response, complement, hypoxia, NF-kB, IL6 and IL2-mediated signaling. In contrast, in the BxPC3-R/BxPC3 cell pair, many of these same gene sets were enriched in the parental phenotype but not in the resistant phenotype (Supplementary Material, Supplementary Figs. S3-S6). In addition, gene sets relevant to KRAS signaling that are enriched in AsPC1-R resistant cells display enrichment in BxPC3 parental cells. Overall the GSEA indicates that, relative to parental cells, AsPC1-R cells display expression changes consistent with activated EGFR, KRAS, RAF and AKT and reduced activity of MYC, E2F and NOTCH, whereas the expression profile of BxPC3-R cells is consistent with reduced activity of EGFR, KRAS, RAF and AKT and enhanced activity of MYC, E2F and NOTCH.
Cisplatin-resistant AsPC1-R and BxPC3-R cells display changes in gene expression for different cisplatin resistance genes
Previous studies have identified genes directly associated with resistance to cisplatin therapy (Supplementary Material, Supplementary Table S1). These genes are involved in functionally significant processes such as drug transport, drug inactivation, DNA damage response, DNA repair and apoptosis. We selected 72 of the best characterized of these genes (Supplementary Material, Supplementary Table S1) to compare their expression patterns in our resistant and parental cell lines. Twenty of these genes displayed significant changes in expression in at least one of the resistant cell lines relative to their sensitive parental controls (Table 2). We have validated the results of our microarray analysis and confirmed that CCND1 (cyclin D1) is upregulated in AsPC1-R cells; ERCC1 (excision repair cross-complementation group 1) is upregulated in BxPC3-R cells and CLU (clusterin) is upregulated in both AsPC1-R and BxPC3-R cells relative to corresponding parental cell lines (Supplementary Method and Results).
Table 2. Genes implicated in cisplatin resistance and significantly differentially expressed in at least one pair of sensitive/resistant pancreatic cancer cell lines.
| Gene | FC (AsPC1-R/S) | FC (BxPC3-R/S) | FC (BxPC3/AsPC1) | Mode of action in the resistance to cisplatin ref. (19, 20, 21, 22, 23, 24) |
|---|---|---|---|---|
| ABCC2 | 1.56a | NS | −12.92 | Drug transport |
| ATP7A | NS | 1.63a | −1.88 | Drug transport |
| BCL2A1 | NS | −2.08b | 2.35 | Apoptosis |
| BIRC3 | 2.42a | −4.46b | NS | Apoptosis |
| CCND1 | 1.51a | NS | NS | Cell cycle/apoptosis |
| CD44 | 1.72a | NS | 1.95 | Apoptosis |
| CFLAR | 1.63a | −2.6b | NS | Apoptosis |
| CHEK2 | NS | −1.55a | NS | DNA damage response |
| CLU | 2.21a | 2.75a | NS | Apoptosis |
| ERCC1 | NS | 1.63a | NS | Nucleotide excision repair |
| GCLC | 1.9a | −2.2b | 1.66 | Drug inactivation |
| GCLM | 2.23a | NS | 3.88 | Drug inactivation |
| GSTT2 | 1.55a | NS | NS | Drug inactivation |
| HIST1H1A | −2.07b | NS | 3.96 | Unknown |
| LRRFIP1 | 1.67a | 2.01a | NS | Drug accumulation |
| MT2A | −2.96b | NS | 8.50 | Drug inactivation |
| MVP | NS | −1.94b | −1.61 | Pleiotropic / drug transport |
| NQO1 | NS | −1.76b | NS | Pleiotropic / ROS removal |
| POLB | 1.72a | NS | NS | DNA translesion synthesis |
| SLC31A2 | 1.55a | NS | 2.07 | Drug transport |
Abbreviations: NS, not significant; ROS, reactive oxygen species. FC(AsPC1-R/S), FC(BxPC3-R/S): Expression fold change between corresponding resistant/parental cells; FC(BxPC3/AsPC1): Expression fold change between two parental cell lines
gene displays expression changes consistent with acquisition of resistant phenotype.
Gene displays expression changes inconsistent with acquisition of resistant phenotype. Difference in the expression between parental cell lines is indicated in the 4th column ‘FC (BxPC3 vs AsPC1)'.
Although both pairs of resistant/parental PDAC cell lines displayed significant changes in gene expression that were consistent with the acquisition of cisplatin resistance, only two genes, CLU and LRRFIP1 (Leucine Rich Repeat Interacting Protein 1), were significantly overexpressed in both resistant cell lines relative to their parental cell lines.
The majority of changes in expression of genes previously associated with cisplatin resistance were observed in one or the other of the selected cell lines but not both. For example, ERCC1, POLB (polymerase (DNA directed), beta) and CHEK2 (checkpoint kinase 2) are genes previously shown to be involved in nucleotide excision repair29 and DNA translesion synthesis.30 Expression of ERCC1 was significantly increased in BxPC3-R cells but not in AsPC1-R cells, whereas expression of POLB was significantly increased in AsPC1-R cells but not in BxPC3-R cells, relative to parental cell lines. Thus, the development of cisplatin resistance in both cell lines appears to have involved the acquisition of enhanced DNA repair processes but by alternative strategies.
In some cases, the results suggest that the alternative strategies may have been influenced by differences in gene expression levels pre-existing between the two parental cell lines. For example, the drug transporter-encoding gene ATP7A (ATPase, Cu2+ transporting, alpha polypeptide) is expressed at a significantly higher level in parental AsPC1 cells relative to parental BxPC3 cells (Table 2). Likewise, ABCC2 (ATP-Binding Cassette Sub-family C Member 2) and MVP/LRP (Major Vault Protein/Lung Resistance-Related Protein) genes are upregulated in parental AsPC1 cells, and these starting differences may, in part, explain why AsPC1 cells are inherently more resistant to cisplatin than BxPC3 cells (IC50: 20 μM vs 0.9 μM, respectively). Interestingly, the acquisition of cisplatin resistance resulted in a significant increase in ATP7A expression levels in BxPC3-R cells but not in AsPC1-R cells possibly because the gene was already expressed at a relatively high level in the AsPC1 parental cell line (Table 2).
Similar reasoning may apply to the significant increases in mRNA levels of the CD44 (CD44 molecule-Indian blood group), GCLM (Glutamate-Cysteine Ligase, Modifier Subunit) and SLC31A2 (Solute Carrier Family 31, Member 2) genes in AsPC1-R cells but not in BxPC3-R cells relative to their parental controls.
In a number of instances, there was a clear preference for increased expression of cisplatin resistance genes by one cell line or the other regardless of differences in expression between parental cell lines. For example, increased expression of the ABCC2 (ATP-Binding Cassette, Sub-Family C, Member 2), BIRC3 (Baculoviral IAP Repeat Containing 3) and CFLAR (CASP8 And FADD-Like Apoptosis Regulator) genes was associated with the cisplatin-resistant AsPC1-R cells but not with BxPC3-R cells. Conversely, reduced expression of the HIST1H1A (Histone Cluster 1, H1a) and MT2A (Metallothionein 2A) genes was found in AsPC1-R cells but not in BxPC3-R cells.
In a few cases, the two cell line pairs displayed differences in the direction of expression changes associated with increased resistance to cisplatin. For example, expression of BIRC3 gene, although significantly increased in the resistant AsPC1-R cells, was significantly reduced in the resistant BxPC3-R cells all relative to their respective parental cell lines. The downregulation of cisplatin resistance genes in cells developed for increased resistance seems counterintuitive but again indicates apparent selection for alternative molecular pathways in the acquisition of resistance in the two cell lines. The unexpected downregulation of some cisplatin resistance genes in one or other of the selected cell lines may simply reflect stochastic changes in the expression of some genes or signaling pathways not directly involved in the adaptive response of a particular cell line.
Discussion
Pancreatic cancer, most commonly represented by PDAC, is one of the most lethal malignancies with 46 420 new cases and 39 590 deaths estimated in the USA for 2014.31 The traditional antineoplastic agent cisplatin has demonstrated remarkable efficacy in the treatment of testicular germ cell tumors and has been shown to provide significant clinical benefits in the treatment of various other cancers.32 Currently cisplatin is being evaluated in combination chemotherapy for PDAC in several clinical trials (https://clinicaltrials.gov/). As PDAC displays limited response to current chemotherapies due to a number of reasons, including, most frequently, intrinsic and acquired drug resistance,33 clinical efficacy of cisplatin may be limited by drug resistance as well.
Extensive prior research has demonstrated that cancer cells can develop resistance to cisplatin in a variety of ways (reviewed in refs 13, 20, 21, 34) that include (i) pre-target resistance, for example, by reduced accumulation or increased extrusion of cisplatin by transporters, or its enhanced inactivation by glutathione (GSH) or metallothionein-mediated sequestration; (ii) on-target resistance, through DNA repair; (iii) post-target resistance via modulation of DNA damage recognition, damage response and apoptosis; and, (iv) off-target resistance, for example, through compensatory pro-survival signals or unspecific adaptive responses not directly activated by cisplatin.34
Although cisplatin-resistant cells with a single major mechanism responsible for their drug resistance have been reported,35 in the majority of cases, cisplatin resistance appears to be multifactorial with several unrelated mechanisms employed simultaneously within the same cell.36
The purpose of the present study was to independently develop cisplatin resistance in two distinct parental PDAC cell lines and to subsequently examine the molecular mechanisms associated with the acquired resistance. We primarily focused on comparison of pathways enriched by differentially expressed genes, as well as gene sets enriched in cisplatin-resistant or parental phenotypes. In addition, we have examined changes in expression of 72 genes previously found to be involved in cisplatin resistance of cancer cells (Supplementary Material, Supplementary Table S1). Our study included only microarray analysis without functional validation of the role of specific differentially expressed genes in the resistance of cancer cells to cisplatin. Nevertheless, this analysis is sufficient to examine how different cell lines of the same cancer type deploy different combinations of known cisplatin resistance-associated genes to achieve drug-resistant phenotype.
The cell lines selected for our study represent in vitro models of PDAC with considerably different origin, molecular properties and morphology in cell culture. Specifically, AsPC1 cells are K-Ras mutant cells isolated from ascites of a poorly differentiated metastatic carcinoma of the head of the pancreas, whereas BxPC3 is a K-Ras wild-type cell line developed from a moderately-to-poorly differentiated primary adenocarcinoma of the body of pancreas (Supplementary Material, Supplementary Table S2). In addition, these two cell lines differ in mutational status of other cancer relevant genes and expression of pro-angiogenic factors.37 These considerably different PDAC cell lines were selected to represent diversity that can be encountered in this tumor type and development of its resistance to cisplatin. Other investigators have also used these distinct cell lines in a gene expression study to represent variability of pancreatic adenocarcinomas and their response to cell invasion-inducing agent phorbol 12-myristate 13-acetate.38 Likewise, these cell lines have been used in a functional study of the role of Twist and GDF15 genes in cisplatin resistance.39
In general, our findings support a multifactorial character for cisplatin resistance developed in two different PDAC cell lines that include mechanisms related to drug transport, drug inactivation, DNA damage response, DNA repair and the modulation of apoptosis.
The results of our molecular pathway analyses of the two pairs of parental/resistant cell lines indicate that although cellular functions previously associated with drug resistance were significantly changed in both cell lines, alternative strategies were taken in acquiring resistance. For instance, K-Ras signaling is activated in AsPC1-R but not in BxPC3-R cells, whereas MYC-signaling is activated in BxPC3-R but not in AsPC1-R cells (Supplementary Material, Supplementary Figures S3 and S4).
A similar result was observed when our analysis focused on specific genes previously associated with cisplatin resistance. Although many of these previously identified genes displayed significant changes in expression in the resistant cell lines, many of these changes were found to be unique to one cell line or the other. Specifically, the cisplatin resistance-related genes ABCC2, BIRC3, CCND1, CFLAR, CHEK2, ERCC1, MT2A, POLB, GCLC and GSTT2 were found likely to be involved in the resistance of one but not both cisplatin-resistant PDAC cell lines. The ABCC2 gene codes for MRP2/cMOAT transporter that confers resistance to cisplatin by enhanced efflux of GSH-cisplatin conjugates.40 BIRC3 (c-IAP2) is a member of the Inhibitors of Apoptosis Proteins (IAP) family that inhibits caspase activity.41 CCND1 likely contributes to cisplatin resistance both through cell cycle control and inhibition of apoptosis.42 CFLAR (c-FLIP) inhibits apoptosis by blocking the activation of caspase-8.43 The CHEK2 gene encodes for a Chk2 kinase involved in the propagation of the DNA damage signal affecting cell cycle arrest, DNA repair and apoptosis, and its degradation or decreased expression is associated with cisplatin resistance.44 ERCC1 is a component of ERCC1-XPF endonuclease that has a critical role in repair of cisplatin-induced DNA lesions by nucleotide excision repair. Furthermore, ERCC1-XEF facilitates repair of double strand breaks induced by processing of cisplatin-induced DNA interstrand crosslinks.45 MT2A, an intracellular cysteine-rich protein, inactivates cisplatin through interaction and scavenging of reactive oxygen species generated in cisplatin-treated cells (reviewed in 13). POLB is an error-prone DNA polymerase involved in base excision repair, and its upregulation enhances genetic instability and cisplatin resistance.46 Both GCLC and GSTT2 are involved in GSH homeostasis and contribute to cisplatin resistance through inactivation of cisplatin and reactive oxygen species scavenging.21
Likewise, AsPC1-R but not BxPC3-R cells display activated NF-kB signaling that has been previously shown to inhibit cisplatin-induced apoptosis in some but not all examined cancer cell lines.23 NF-kB, which has a complex signaling role in inflammation and cancer, likely acts through transcriptional activation of negative regulators of apoptosis, including CFLAR47 and BIRC3.48 Both these genes were upregulated in AsPC1-R but not BxPC3-R cells.
Our conclusion from pathway enrichment analysis that NF-kB signaling is activated in AsPC1-R cells is further supported by upregulation of CCND1, which is known to be a direct transcriptional target of NF-kB.49 CCND1 was confirmed to be overexpressed in AsPC1-R cells relative to AsPC1 cells by quantative PCR expression assay (Supplementary Methods).
The resistant cell lines AsPC1-R and BxPC3-R also differ in the activity of MYC and EGFR/RAS/MAPK pathway, which can, depending on the cell context, contribute to the cisplatin resistance through compensatory pro-survival signals.36 Oncoprotein c-MYC has been recently shown to promote cisplatin resistance through increased activity of PARP1, a key component of base excision repair.50 Activated EGFR/RAS/MAPK signaling reportedly enhances cisplatin resistance by increased GSH levels and upregulation of transcriptional targets of AP1 complex, including GST2 gene.21
Differences between parental cell lines AsPC1 and BxPC3 in their sensitivities to cisplatin could be attributed to upregulation of ABCC2, ATP7A and MVP/LRP transporters, which are known to mediate cellular efflux of cisplatin or cisplatin-glutathione conjugate.50, 51, 52, 53 Upregulation of these cisplatin resistance genes in AsPC1 cells relative to BxPC3 cells was also found in the GSE22973 data set (Gene Expression Omnibus; www.ncbi.nlm.nih.gov/geo), which supports the validity of our gene expression data (Supplementary Material, Supplementary Figure S7).
Our results suggest that the alternative strategies taken by the two resistant cell lines may be at least partially dictated by molecular differences between the parental cell lines. For example, activation of EGFR/RAS/KRAS signaling in AsPC1-R cells likely reflects the presence of oncogenic activating mutation G12D in KRAS gene.17 In other cases, however, the alternative strategies may simply be a reflection of initial random selection of one or a subset of alternative adaptive responses that become subsequently reinforced over repeated rounds of selection.
Only two cisplatin resistance-related genes CLU and LRRFIP1 were found upregulated in both resistant cell lines. This finding is consistent with previously reported results that demonstrated few consistently differentially expressed genes across several parental/resistant pairs of cells derived from other cancer type.54 Secreted isoform of CLU confers anticancer drug resistance through its interaction with activated Bax and subsequent inhibition of apoptosis.22 Although CLU has been previously associated with resistance of pancreatic cancer cells to gemcitabine,55 its involvement in cisplatin resistance of pancreatic cancer cells had not yet been reported. As a result, our finding is intriguing and extends understanding of the role of CLU in drug resistance of pancreatic cancer cells.
The complexity of network interactions that underlie cellular functions provides cancer cells with a variety of alternative strategies by which to respond or adapt to environmental and/or mutational changes over time. Indeed, it is this inherent cellular complexity that is believed to underlie the molecular individuality of pancreatic56 and other types of cancers57 and which supports the implementation of personalized approaches to cancer therapy.58 Our results indicate that the resistance of pancreatic and perhaps other cancer cells to cisplatin may also be attained by a variety of alternative molecular mechanisms/pathways and that strategies to inhibit or reverse acquired resistance of cancer cells to cisplatin cannot be generalized but will require individual molecular profiling and analysis.
Acknowledgments
This work was supported by the Petit Undergraduate Research Scholars Program of Parker H. Petit Institute for Bioengineering and Bioscience and the Georgia Tech Foundation.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cancer Gene Therapy website (http://www.nature.com/cgt)
Supplementary Material
References
- Muniraj T, Jamidar PA, Aslanian HR. Pancreatic cancer: a comprehensive review and update. Dis Mon 2013; 59: 368–402. [DOI] [PubMed] [Google Scholar]
- Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF et al. SEER Cancer Statistics Review (CSR) 1975-2012. National Cancer Institute: Bethesda, MD, USA, 2015. (Accessed on 09 January 2015). http://seer.cancer.gov/csr/1975_2012/. [Google Scholar]
- Riall TS, Lillemoe KD. Underutilization of surgical resection in patients with localized pancreatic cancer. Ann Surg 2007; 246: 181–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie D, Xie K. Pancreatic cancer stromal biology and therapy. Genes Dis 2015; 2: 133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg 1996; 223: 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cid-Arregui A, Juarez V. Perspectives in the treatment of pancreatic adenocarcinoma. World J Gastroenterol 2015; 21: 9297–9316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817–1825. [DOI] [PubMed] [Google Scholar]
- D'Andrea AD, Grompe M. The Fanconi anaemia/BRCA pathway. Nat Rev Cancer 2003; 3: 23–34. [DOI] [PubMed] [Google Scholar]
- van der Heijden MS, Brody JR, Gallmeier E, Cunningham SC, Dezentje DA, Shen D. Functional defects in the fanconi anemia pathway in pancreatic cancer cells. Am J Pathol 2004; 165: 651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch FJ, Johnson MR, Rabe K, Boardman L, McWilliams R, de Andrade M et al. Germ line Fanconi anemia complementation group C mutations and pancreatic cancer. Cancer Res 2005; 65: 383–386. [PubMed] [Google Scholar]
- Berners-Price SJ. Activating platinum anticancer complexes with visible light. Angew Chem Int Ed Engl 2011; 50: 804–805. [DOI] [PubMed] [Google Scholar]
- Kartalou M, Essigmann JM. Mechanisms of resistance to cisplatin. Mutat Res 2001; 478: 23–43. [DOI] [PubMed] [Google Scholar]
- Mezencev R. Interactions of cisplatin with non-DNA targets and their influence on anticancer activity and drug toxicity: the complex world of the platinum complex. Curr Cancer Drug Targets 2015; 14: 794–816. [DOI] [PubMed] [Google Scholar]
- Chen WH, Horoszewicz JS, Leong SS, Shimano T, Penetrante R, Sanders WH et al. Human pancreatic adenocarcinoma: in vitro and in vivo morphology of a new tumor line established from ascites. In Vitro 1982; 18: 24–34. [DOI] [PubMed] [Google Scholar]
- Tan MH, Nowak NJ, Loor R, Ochi H, Sandberg AA, Lopez C et al. Characterization of a new primary human pancreatic tumor line. Cancer Invest 1986; 4: 15–23. [DOI] [PubMed] [Google Scholar]
- Coley HM. Development of drug-resistant models. In: Langdon SP. Cancer Cell Culture Methods and Protocols. Humana Press: Totowa, New Jersey, 2004: 267–273. [DOI] [PubMed] [Google Scholar]
- Mezencev R, Updegrove T, Kutschy P, Repovska M, McDonald JF. Camalexin induces apoptosis in T-leukemia Jurkat cells by increased concentration of reactive oxygen species and activation of caspase-8 and caspase-9. J Nat Med 2011; 65: 488–499. [DOI] [PubMed] [Google Scholar]
- Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 2001; 98: 5116–5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CC-K. A search for the genes involved in the resistance to cisplatin chemotherapy: Review of the experimental evidence. Curr Topics Pharmacol 2010; 14: 47–54. [Google Scholar]
- Shen DW, Pouliot LM, Hall MD, Gottesman MM. Cisplatin resistance: a cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol Rev 2012; 64: 706–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddik ZH. Cisplatin resistance. Molecular basis of multifaceted impediment. In: Teicher B. Cancer Drug Discovery and Development. Humana Press: Totowa, NJ, USA, 2006: 283–307. [Google Scholar]
- Djeu JY, Wei S. Clusterin and chemoresistance. Adv Cancer Res 2009; 105: 77–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart DJ. Mechanisms of resistance to cisplatin and carboplatin. Crit Rev Oncol Hematol 2007; 63: 12–31. [DOI] [PubMed] [Google Scholar]
- Fujimori S, Abe Y, Nishi M, Hamamoto A, Inoue Y, Ohnishi Y et al. The subunits of glutamate cysteine ligase enhance cisplatin resistance in human non-small cell lung cancer xenografts in vivo. Int J Oncol 2004; 25: 413–418. [PubMed] [Google Scholar]
- Shi W, Bessarabova M, Dosymbekov D, Dezso Z, Nikolskaya T, Dudoladova M et al. Functional analysis of multiple genomic signatures demonstrates that classification algorithms choose phenotype-related genes. Pharmacogenomics J 2010; 10: 310–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005; 102: 15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merico D, Isserlin R, Stueker O, Emili A, Bader GD. Enrichment map: a network-based method for gene-set enrichment visualization and interpretation. PLoS One 2010; 5: e13984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 2003; 13: 2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry KV, Hamilton TC, Johnson SW. Increased nucleotide excision repair in cisplatin-resistant ovarian cancer cells: role of ERCC1-XPF. Biochem Pharmacol 2000; 60: 1305–1313. [DOI] [PubMed] [Google Scholar]
- Hoffmann JS, Pillaire MJ, Maga G, Podust V, Hubscher U, Villani G. DNA polymerase beta bypasses in vitro a single d(GpG)-cisplatin adduct placed on codon 13 of the HRAS gene. Proc Natl Acad Sci USA 1995; 92: 5356–5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014; 64: 9–29. [DOI] [PubMed] [Google Scholar]
- Chabner BA. Barnett Rosenberg: in memoriam (1924-2009) obituary. Cancer Res 2010; 70: 428–429. [Google Scholar]
- Oberstein PE, Olive KP. Pancreatic cancer: why is it so hard to treat? Therap Adv Gastroenterol 2013; 6: 321–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Vitale I, Michels J, Brenner C, Szabadkai G, Harel-Bellan A et al. Systems biology of cisplatin resistance: past, present and future. Cell Death Dis 2014; 5: e1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelland LR, Mistry P, Abel G, Loh SY, Oneill CF, Murrer BA et al. Mechanism-related circumvention of acquired cis-diamminedichloroplatinum(ii) resistance using two pairs of human ovarian-carcinoma cell-lines by ammine amine platinum(iv) dicarboxylates. Cancer Res 1992; 52: 3857–3864. [PubMed] [Google Scholar]
- Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene 2003; 22: 7265–7279. [DOI] [PubMed] [Google Scholar]
- Deer EL, Gonzalez-Hernandez J, Coursen JD, Shea JE, Ngatia J, Scaife CL et al. Phenotype and genotype of pancreatic cancer cell lines. Pancreas 2010; 39: 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers A, Smith MJ, Doolan P, Clarke C, Clynes M, Murphy JF et al. Invasive markers identified by gene expression profiling in pancreatic cancer. Pancreatology 2012; 12: 130–140. [DOI] [PubMed] [Google Scholar]
- Ji H, Lu HW, Li YM, Lu L, Wang JL, Zhang YF et al. Twist promotes invasion and cisplatin resistance in pancreatic cancer cells through growth differentiation factor 15. Mol Med Rep 2015; 12: 3841–3848. [DOI] [PubMed] [Google Scholar]
- Kawabe T, Chen ZS, Wada M, Uchiumi T, Ono M, Akiyama S et al. Enhanced transport of anticancer agents and leukotriene C4 by the human canalicular multispecific organic anion transporter (cMOAT/MRP2). FEBS Lett 1999; 456: 327–331. [DOI] [PubMed] [Google Scholar]
- Nomura T, Yamasaki M, Nomura Y, Mimata H. Expression of the inhibitors of apoptosis proteins in cisplatin-resistant prostate cancer cells. Oncol Rep 2005; 14: 993–997. [PubMed] [Google Scholar]
- Noel EE, Yeste-Velasco M, Mao X, Perry J, Kudahetti SC, Li NF et al. The association of CCND1 overexpression and cisplatin resistance in testicular germ cell tumors and other cancers. Am J Pathol 2010; 176: 2607–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta H, Eby MT, Gazdar AF, Chaudhary PM. Role of MRIT/cFLIP in protection against chemotherapy-induced apoptosis. Cancer Biol Therapy 2002; 1: 652–660. [DOI] [PubMed] [Google Scholar]
- Zhang P, Gao W, Li H, Reed E, Chen F. Inducible degradation of checkpoint kinase 2 links to cisplatin-induced resistance in ovarian cancer cells. Biochem Biophys Res Commun 2005; 328: 567–572. [DOI] [PubMed] [Google Scholar]
- Arora S, Kothandapani A, Tillison K, Kalman-Maltese V, Patrick SM. Downregulation of XPF-ERCC1 enhances cisplatin efficacy in cancer cells. DNA Repair 2010; 9: 745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudsocq F, Benaim P, Canitrot Y, Knibiehler M, Ausseil F, Capp JP et al. Modulation of cellular response to cisplatin by a novel inhibitor of DNA polymerase beta. Mol Pharmacol 2005; 67: 1485–1492. [DOI] [PubMed] [Google Scholar]
- Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J. NF-kappa B signals induce the expression of c-FLIP. Mol Cell Biol 2001; 21: 5299–5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS. NF-kappa B antiapoptosis: Induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 1998; 281: 1680–1683. [DOI] [PubMed] [Google Scholar]
- Toualbi-Abed K, Daniel F, Guller MC, Legrand A, Mauriz JL, Mauviel A et al. Jun D cooperates with p65 to activate the proximal kappaB site of the cyclin D1 promoter: role of PI3K/PDK-1. Carcinogenesis 2008; 29: 536–543. [DOI] [PubMed] [Google Scholar]
- Cregan IL, Dharmarajan AM, Fox SA. Mechanisms of cisplatin-induced cell death in malignant mesothelioma cells: Role of inhibitor of apoptosis proteins (IAPs) and caspases. Int J Oncol 2013; 42: 444–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarimboli G. Membrane transporters as mediators of cisplatin effects and side effects. Scientifica 2012; 2012: 473829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K, Wada M, Kohno K, Nakamura T, Kawabe T, Kawakami M et al. A human canalicular multispecific organic anion transporter (cmoat) gene is overexpressed in cisplatin-resistant human cancer cell lines with decreased drug accumulation. Cancer Res 1996; 56: 4124–4129. [PubMed] [Google Scholar]
- Berger W, Spiegl-Kreinecker S, Buchroithner J, Elbling L, Pirker C, Fischer J et al. Overexpression of the human major vault protein in astrocytic brain tumor cells. Int J Cancer 2001; 94: 377–382. [DOI] [PubMed] [Google Scholar]
- Cheng TC, Manorek G, Samimi G, Lin X, Berry CC, Howell SB. Identification of genes whose expression is associated with cisplatin resistance in human ovarian carcinoma cells. Cancer Chemother Pharmacol 2006; 58: 384–395. [DOI] [PubMed] [Google Scholar]
- Chen Q, Wang Z, Zhang K, Liu X, Cao W, Zhang L et al. Clusterin confers gemcitabine resistance in pancreatic cancer. World J Surg Oncol 2011; 9: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lili LN, Matyunina LV, Walker LD, Daneker GW, McDonald JF. Evidence for the importance of personalized molecular profiling in pancreatic cancer. Pancreas 2014; 43: 198–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell RA, McGranahan N, Bartek J, Swanton C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature 2013; 501: 338–345. [DOI] [PubMed] [Google Scholar]
- Du W, Elemento O. Cancer systems biology: embracing complexity to develop better anticancer therapeutic strategies. Oncogene 2015; 34: 3215–3225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.