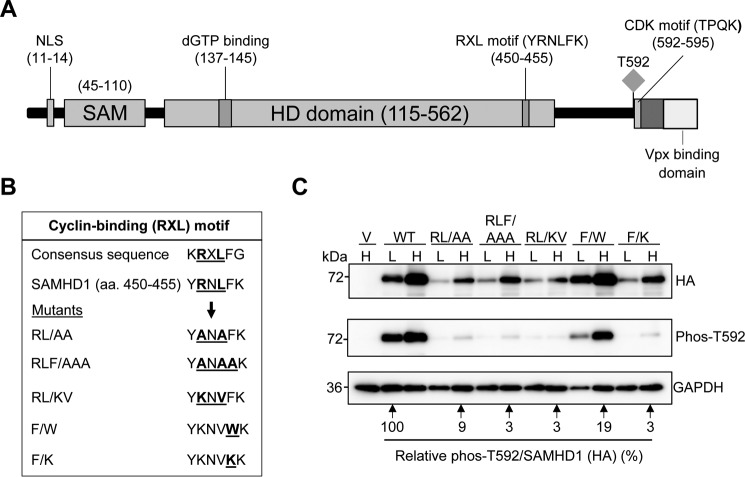
FIGURE 2.
A cyclin-binding (RXL) motif in SAMHD1 decreases SAMHD1 protein expression and Thr592 phosphorylation. A, schematic of the SAMHD1 protein highlighting key domains and residues. The RXL motif (YRNLFK) is in the C-terminal region of the HD domain and positions 450–455. The Thr592 phosphorylation site, the CDK consensus motif (TPQK), and the VPx-binding domain are indicated. B, sequence alignment of the WT SAMHD1 RXL motif with a consensus cyclin-binding motif is shown. Mutations of the conserved residues are also shown. C, immunoblot analysis of HEK293T cells overexpressing HA-tagged WT or SAMHD1 mutants. The cells were transfected with plasmid DNA, and the lysates were harvested 24 h post-transfection. Plasmid DNA input was low (L) at 0.25 μg and high (H) at 2.5 μg. The empty vector was used as a negative control. All transfections were normalized to the same total amount of DNA using empty vector. Membranes were probed for HA and Thr(P)592 SAMHD1. GAPDH was used as a loading control. Densitometry was performed using ImageJ software. Overexpressed HA-tagged SAMHD1 and phospho-SAMHD1 probed membranes were normalized to GAPDH, and the relative amount of phospho-SAMHD1 per total SAMHD1 (HA) was calculated. WT low (L) was set as 100%, and everything was calculated relative to this. Lysates from three independent transfections were used. The data shown are from one representative experiment.