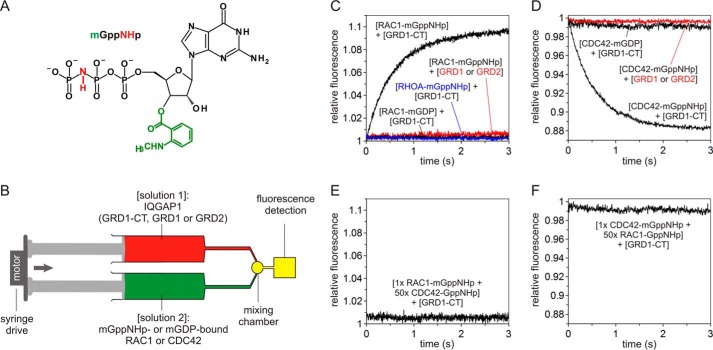
FIGURE 2.
GRD1-CT but not GRD selectively associates only with mGppNHp-bound, active RAC1 and CDC42. A, chemical structure of mGppNHp, a fluorescently labeled, non-hydrolyzable GTP analog, used in this study. B, the stopped-flow device. The stopped-flow device consists of two motorized, thermostated syringes, a mixing chamber, and a fluorescence detector. Two different protein solutions indicated in brackets were rapidly mixed and transferred to a fluorescence detection cell within <4 ms. mGppNHp- or mGDP-bound RHO proteins were used in this study as the fluorescent reporter groups. C and D, association of GRD1-CT with active, mGppNHp-bound CDC42 and RAC1. Kinetics of association were followed by rapidly mixing 2 μm GRD1-CT, GRD1, or GRD2 with 0.2 μm mGppNHp- or mGDP-bound RAC1 (C) or CDC42 (D). The obtained data are the average of four to six independent measurements. The kobs values obtained for the association of GRD1-CT with mGppNHp-bound CDC42 and RAC1 were 1.68 and 1.80 s−1, respectively. No change in fluorescence was observed for GRD1-CT- and mGDP-bound RAC1 or CDC42 (black), GRD1 or GRD2 with mGppNHp-bound RAC1 or CDC42 (red), and GRD1-CT with RHOA-mGppNHp (blue). E and F, overlapping binding sites of CDC42 and RAC1 for GRD1-CT. Association of RAC1-mGppNHp (0.2 μm) with GRD1-CT (2 μm) was blocked in the presence of excess amount of non-fluorescent CDC42-GppNHp (10 μm) (E). Association of CDC42-mGppNHp (0.2 μm) with GRD1-CT (2 μm) was blocked in the presence of excess amount of non-fluorescent RAC1-GppNHp (10 μm) (F).