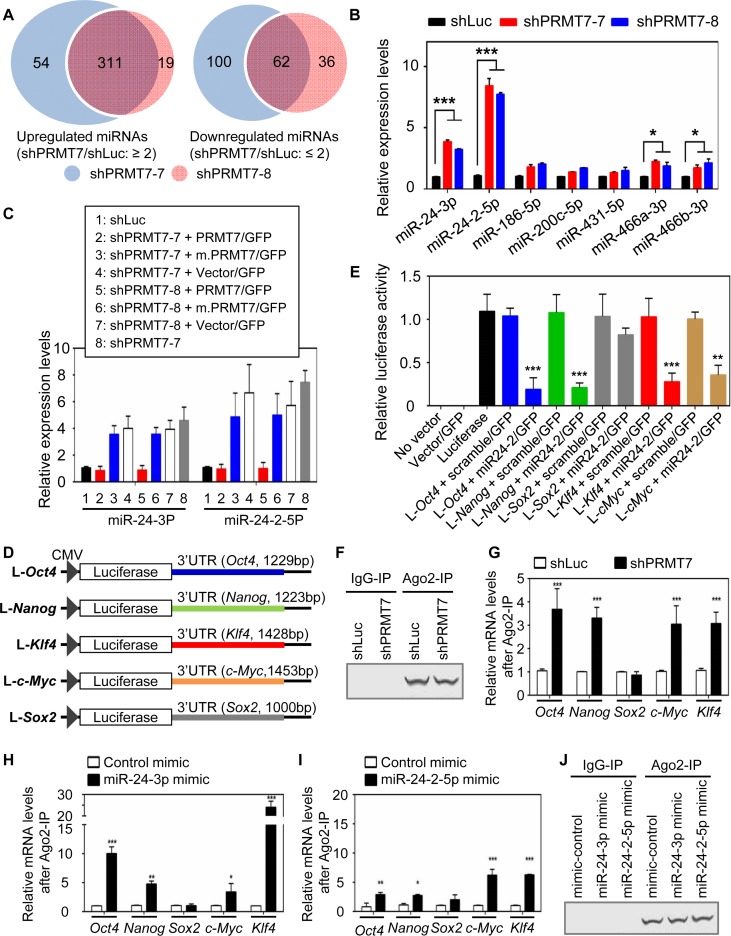
Figure 2.
PRMT7 downregulates the levels of miR-24-3p and miR-24-2-5p, which collectively target the 3′UTRs of Oct4, Nanog, Klf4 and c-Myc mRNAs. (A) Venn diagrams of miRNAs that were significantly upregulated or downregulated by two shPRMT7s (shPRMT7-7 and shPRMT7-8). (B) Comparison of cellular levels of multiple miRNAs between shLuc-treated and PRMT7-depleted V6.5 mESCs using quantitative, miRNA-specific PCR. (C) The effect of ectopic expression of PRMT7 or its catalytic mutant (m.PRMT7) on miR-24-3p and miR24-2-5p levels in PRMT7-depleted mESCs. (D) Schematic representation of luciferase reporter constructs containing Oct4-3′UTR, Nanog-3′UTR, Sox2-3′UTR, Klf4-3′UTR or c-Myc-3′UTR. (E) Relative luciferase activities of reporter constructs containing Oct4-3′UTR, Nanog-3′UTR, Sox2-3′UTR, Klf4-3′UTR or c-Myc-3′UTR in the absence or presence of miR24-2 expression. The reporter constructs, alone or together with a miR-24-2 expression plasmid encoding miR-24-3p and miR24-2-5p, were transfected into HEK293T cells. Firefly luciferase activities were normalized to the internal transfection control Renila luciferase. (F and G) Comparison of endogenous association of Ago2 with Oct4, Nanog, Sox2, Klf4 and c-Myc mRNAs between shLuc-treated cells and PRMT7-depleted cells. Ago2 IP was performed (F) and IP eluates were analyzed using quantitative RT-PCR (G). (H−J) Analysis of the association of Ago2 with Oct4, Nanog, Sox2, Klf4 and c-Myc mRNAs after transient transfection of control mimic, miR-24-3p mimic (H) or miR-24-2-5p mimic (I) in mESCs. Following transfection of mimic RNAs, cells were incubated for 48 h, lysed and used for Ago2 IP (J). IP eluates were analyzed using quantitative RT-PCR (H and I). Data are presented as the mean ± SD of three independent experiments. P < 0.05 (*), P < 0.01 (**) and P <0.001 (***) indicate statistically significant changes.