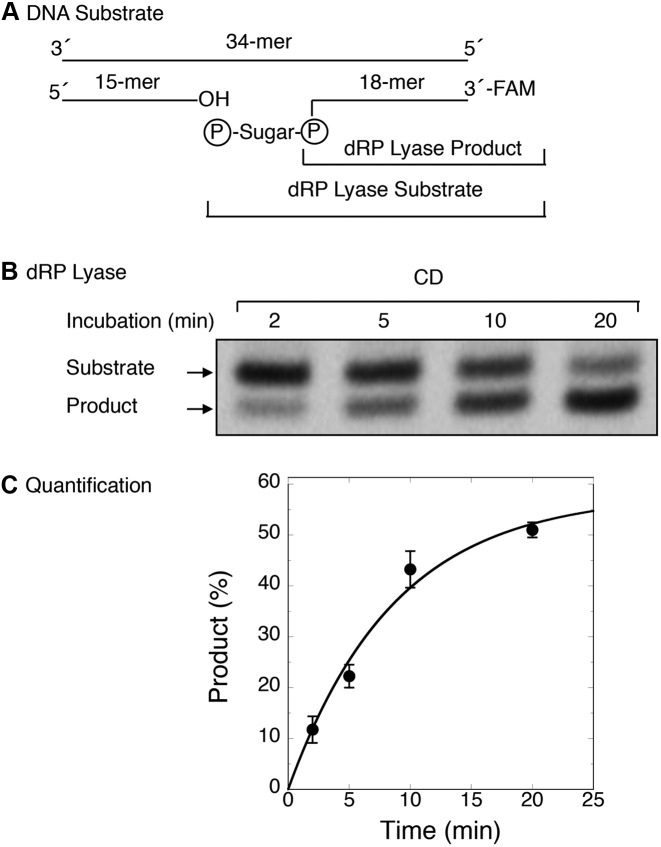
Figure 5.
Kinetic measurements of dRP lyase activity of the CD of Rev1. The release of dRP from the incised AP-site containing DNA substrate was examined as a function of incubation time, and the rate of dRP removal by CD was determined under single turnover conditions (i.e. enzyme/DNA = 5). The reaction was performed with purified CD as described under ‘Materials and Methods’. (A) Schematic representation of the DNA substrate prepared by annealing a 15-mer oligonucleotide, a 18-mer oligonucleotide containing uracil at the 5′-end in the nick and FAM at the 3′-end, and a 34-mer oligonucleotide template. (B) The duplex DNA (100 nM) was added to a reaction mixture with 500 nM CD. Aliquots were withdrawn at the indicated times, and the products were stabilized by the addition of 20 mM NaBH4 and incubation on ice for 30 min. Then, an equal volume of gel-loading dye buffer was added, and the reaction products were analyzed as in Figure 2B. A representative phosphorimage of the polyacrylamide gel illustrates product formation at various times. (C) Time course data were analyzed using ImageQuant software and fitted to an exponential equation by using nonlinear least squares methods to determine the reaction rate (kobs = 0.10 per minute). Average values of relative product formed (%) from four experiments were plotted against incubation time (min). Standard error bars are shown.