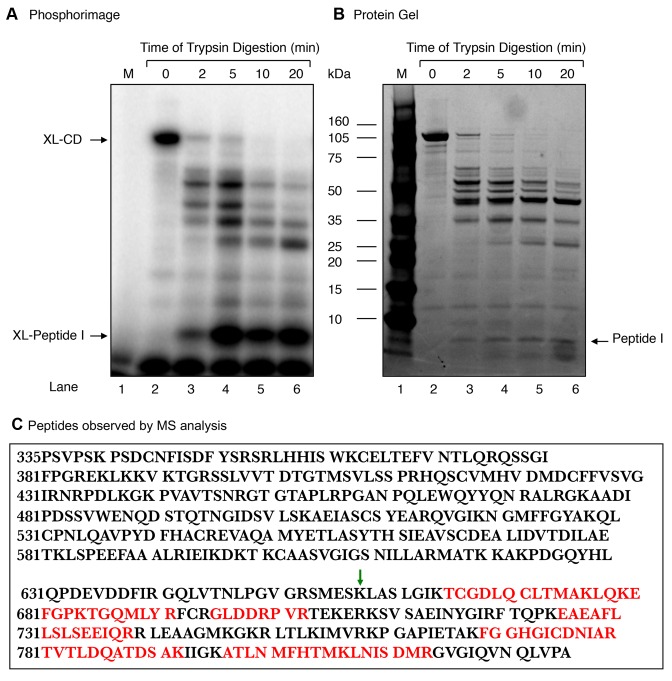
Figure 6.
Limited proteolysis of the Rev1 CD with trypsin. Purified 100-kDa CD (66 μg) was digested at 25°C with trypsin (0.66 μg) at a weight ratio of 1:100 (trypsin:CD) in 100 mM Tris–HCl, pH 8.0. Aliquots were withdrawn at 2, 5, 10 and 20 min, as indicated at the top of the photograph. (A) A portion (1/10th) of each digested sample was incubated with 5′-end labeled dRP lyase DNA substrate and then subjected to NaBH4 cross-linking as in Figure 2. The gel was scanned on a PhosphorImager. The positions of XL-CD and the ∼XL-peptide (I) (∼8-kDa) are indicated. (B) A photograph of the SimplyBlue stained gel in (A) is shown. (C) The radiolabeled fragment corresponding to the ∼ 8-kDa peptide (I) from the stained gel in (B) was incised from the gel and subjected to MS analysis. The tryptic peptide amino acid sequences found in MS/MS analysis, corresponding to the ∼170 amino acid segment of the carboxy-terminus of the CD of Rev1, are indicated in red. The green arrow indicates the deletion site in the mutant CD (MCD) of Rev1 (see Figure 7).