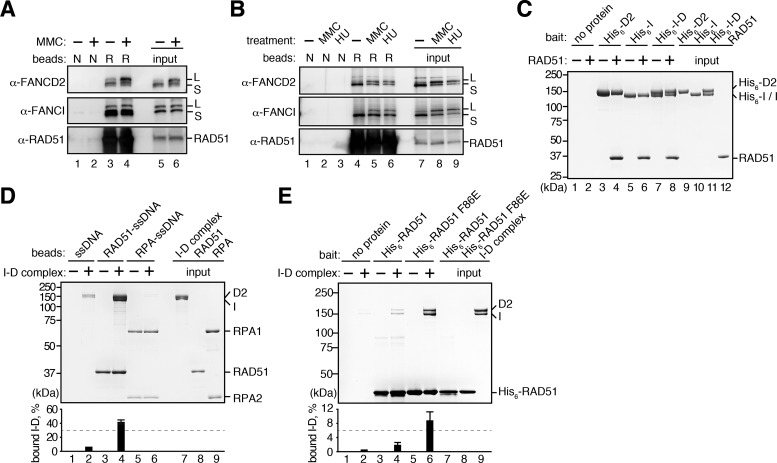
Figure 2.
FANCD2 and FANCI interact with RAD51. (A) Pull-down assay with the HeLa cell extracts. The beads without RAD51 (N) or with RAD51 (R) were incubated with the nuclease-treated cell extracts, and the proteins bound to the beads were detected by Western blotting. The cell extracts were prepared from untreated and MMC-treated HeLa cells. (B) Pull-down assay with the DT40 cell extracts. The nuclease-treated cell extracts were prepared from untreated, MMC-treated and HU-treated DT40 cells. (C) Pull-down assay with Ni-NTA beads. RAD51 bound to His6-tagged FANCI, FANCD2 and the I-D complex was copelleted with the Ni-NTA beads, and the proteins were analyzed by SDS-PAGE. (D) Pull-down assay with single-stranded DNA (ssDNA) beads. The beads conjugated with ssDNA, the RAD51-ssDNA complex or the RPA-ssDNA complex were incubated with the I-D complex. The I-D complex that copelleted with the beads was analyzed by SDS-PAGE. The amounts of the I-D complex in the bound fractions were quantitated, and the mean percentages of three independent experiments are indicated with the standard deviations. (E) Pull-down assay with His6-tagged RAD51 proteins. The I-D complex was copelleted with His6-RAD51 or His6-RAD51 F86E bound to Ni-NTA beads, and the proteins were analyzed by SDS-PAGE with Coomassie Brilliant Blue staining. The amounts of the I-D complex in the bound fractions were quantitated, and the mean percentages of three independent experiments are indicated with the standard deviations.