FIGURE 3.
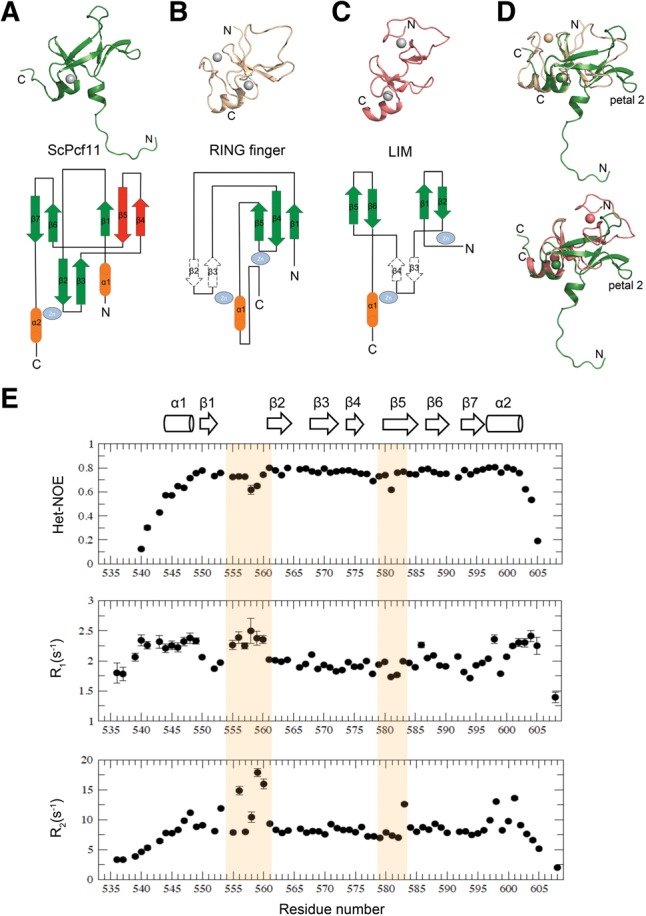
Structural comparison of ScPcf11 538–608 with RING finger and LIM domain proteins. (A) Representative structure of ScPcf11 538–608 (upper) and topology representation (lower). The uncommon “petal” is colored in red. (B) Structure (upper) and topology diagram of a RING finger protein (pdb #1x4j). (C) Structure (upper) and topology diagram (lower) of a LIM protein (pdb #2o13). (D) Alignment of ScPcf11 538–608 (green) with 1x4j (wheat) and with 2o13 (salmon) based on their common structural elements, as described in the main text. (E) Backbone relaxation data of ScPcf11 538–608. Heteronuclear {1H}–15N NOE values (top panel), 15N longitudinal relaxation rate constants R1 (middle panel), and 15N transverse relaxation rate constants R2 (bottom panel) plotted as a function of residue number. Corresponding secondary structures are presented on the top as a column for α helix and an arrow for β strand.
