Fig. 2. Titration of apo-K346 and mdADP.
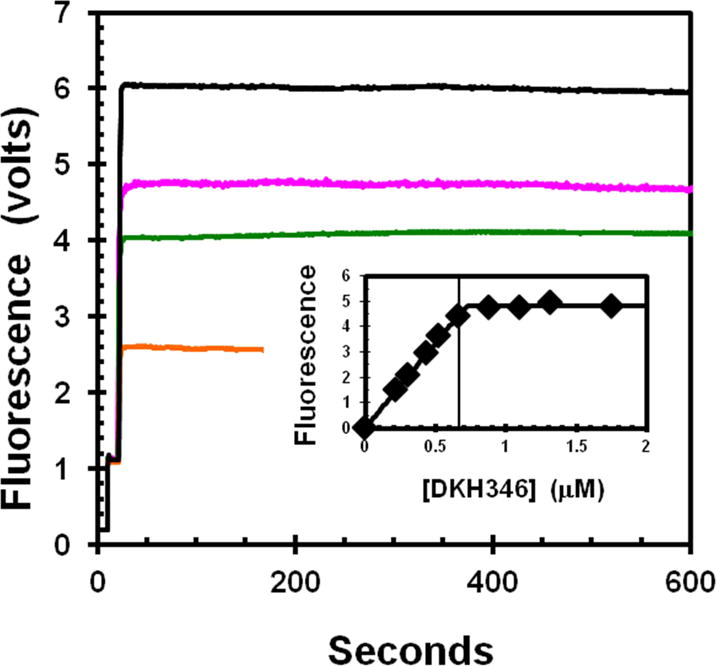
The increase in fluorescence due to FRET from enzyme tyrosines to bound mdADP was determined for addition of varying amounts of apo-K346 from stabilization buffer into a stirred cell containing a fixed concentration of 0.67 μM mdADP in 20% glycerol. Traces begin with buffer only, followed by addition of mdADP at 10 seconds and then apo-K346 at 20 seconds to 0.22, 0.44, 0.53 and 1.31 μM for bottom to top trace respectively. Insert is dependence of the fluorescence increase on the apo-K346 concentration. Small corrections were applied for the fluorescence of apo-K346 alone. The vertical line is at the concentration of mdADP. The theoretical plot is for an unrestrained fit to the full quadratic equation for mutual depletion that gives an equivalence point at 0.72 μM. Insert contains data for addition concentrations of apo-K346 that were not included in the main figure for clarity.
