Figure. 3. Inactivation of MT-ATPase of active apo-K346 by dilution without glycerol.
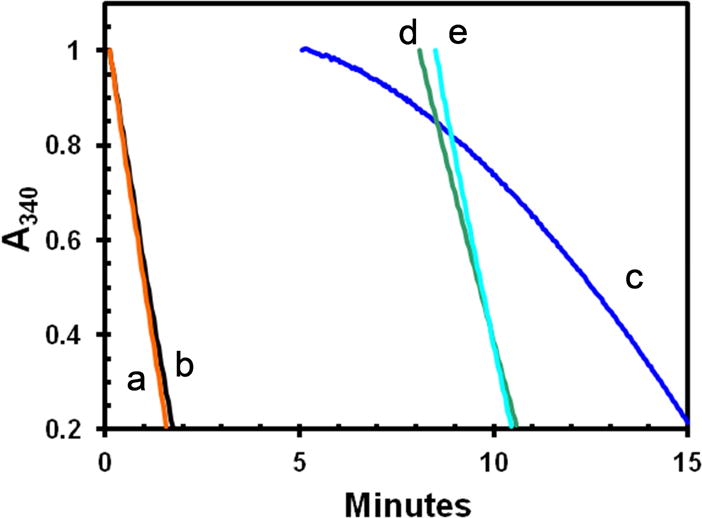
Stocks of E•ADP and apo-K346 in 50% glycerol were prepared at the same concentration and then diluted 100-fold into a complete reaction mixture (25 mM KCl) with taxol and the ATPase rate monitored by loss of absorbance of NADH at 340 nm as previously described [21] using the coupled enzyme system of PEP, pyruvate kinase and lactic dehydrogenase. Curves a and b were initiated by addition of 0.36 μM E•ADP or apo-K346 respectively to a complete reaction mix containing 1 mM MgATP and 1.0 μM MTs. Curve c was initiated by dilution of 0.36 μM apo-K346 into 0.9 volumes of buffer only for 5 minutes, followed by addition of a 10-fold concentrated stock containing ATP, MTs and the other reaction components. Curve d is for dilution of apo-K346 into buffer with 50 μM ATP only for 8 minutes followed by addition of MTs and ATP to 1 mM. Curve e is for dilution of apo-K346 into buffer with MTs but without ATP for 8.5 minutes before addition of ATP to 1 mM. Small offsets were applied so all curves start at an absorbance of 1.0
