Abstract
Considering the depletion of fossil-fuel reserves and their negative environmental impact, new energy schemes must point towards alternative ecological processes. Efficient hydrogen evolution from water is one promising route towards a renewable energy economy and sustainable development. Here we show a tridimensional electrocatalytic interface, featuring a hierarchical, co-axial arrangement of a palladium/titanium dioxide layer on functionalized multi-walled carbon nanotubes. The resulting morphology leads to a merging of the conductive nanocarbon core with the active inorganic phase. A mechanistic synergy is envisioned by a cascade of catalytic events promoting water dissociation, hydride formation and hydrogen evolution. The nanohybrid exhibits a performance exceeding that of state-of-the-art electrocatalysts (turnover frequency of 15000 H2 per hour at 50 mV overpotential). The Tafel slope of ∼130 mV per decade points to a rate-determining step comprised of water dissociation and formation of hydride. Comparative activities of the isolated components or their physical mixtures demonstrate that the good performance evolves from the synergistic hierarchical structure.
Hydrogen evolution by water electrolysis is a promising route to 'green energy', but efficiency is still an issue. Here, the authors make mixed organic/inorganic hierarchical nanostructures with high hydrogen evolution activity, identifying synergic effects in the material contributing to enhanced efficiency.
Renewable energy running on hydrogen embraces a broad range of current technologies so that the hydrogen transition offers a pathway towards the sustainable goal of a carbon-neutral economy. On one side, hydrogen generated from water electrolysis can be linked to renewable electricity (solar, wind and geothermal) to bio-driven devices (microbial cells and algae plants) and to artificial photosynthesis schemes1,2,3. On the other end, hydrogen, as carbon-neutral energy vector, is pivotal for fuel cell technology, as primary fuel for generators and engines, and as chemical commodity for some major industrial processes for synthetic fertilizer or hydrocarbon productions. Furthermore, in situ H2 production provides a greener and safer approach to drive key selective transformations4, including CO2 reduction for environmental remediation. Therefore, increasing the efficiency of H2 production using water as the primary source, while offering a broad flexibility of production and delivery conditions, is mandatory to allow a transition to hydrogen-based energy schemes. The turning point is to be sought within the hydrogen evolution reaction (HER), occurring by the reduction of water at the electrolyser cathode. The HER efficiency and tunability is mainly dependent on the electrocatalyst package in terms of its composition and morphology. Pt-based cathodes are generally considered as the best choice for HER electrocatalysis, by virtue of a generally low overpotential, high turnover frequency and wide pH range of application3,5,6,7. However, Pt-based catalysts are plagued by the unfavourable market price, together with their facile poisoning by water contaminants and the marked loss of activity at under neutral and alkaline conditions8. Research has therefore turned to Pt-free alternatives, and oxides9, sulfides10,11, nitrides12,13, and phosphides2,14,15,16, either as pure phases or in combination with metals, are being investigated for this purpose under several different conditions17,18. An open problem is still the engineering of innovative catalytic cathodes to tackle efficient HER at neutral pH, as this is required to match the operating conditions of some highly promising technologies such as the microbial electrolysis cells or solar water splitting for bio-inspired artificial photosynthesis19. In all cases, key steps for the overall process are as follows: (i) the separation of HER half-reaction (HER, 2H++2e−=H2) from the anodic counterpart; (ii) a tailored design of the electrocatalytic interface to promote the formation of HER intermediates (Had) via water dissociation at neutral pH (H2O+e−→Had+OH−, Volmer step at pH=7); and (iii) the correct trafficking of reducing and oxidizing species between these two compartments20.
For this purpose, an attractive strategy can be envisaged by a hierarchical design of the catalyst nanoarchitecture21, where selected components are arranged to leverage the expected mechanistic functions, namely: (i) water dissociation; (ii) protons reduction; and (iii) electrons translocation22. Carbon nanotubes (CNTs) are prominent materials because of their high surface areas, electrical conductivity and stability in acidic or basic aqueous solutions23. Their one-dimensional morphology is then especially suitable for electrochemical applications, as they behave like nanowires that can connect the active nanostructures to the electrodes. Indeed, many approaches have shown the benefits of CNTs for electrochemical hydrogen production at low overvoltages24. Furthermore, CNT nanoscaffolds can template the arrangement of the metal-based catalytic interfaces by enhancing the active surface area, while connecting the phase boundaries to improve the electrocatalytic efficiency of the process.
The combination of HER electrocatalyst with metal–oxide interfaces has been proposed to boost the hydrogen evolution coupled with water dissociation in the absence of acidic electrolytes. This idea has been demonstrated in the tailored design of Pt surfaces8, and it is here applied to the concept of Pd/TiO2-based units assembled in hierarchical nanostructures for platinum-free HER. This approach has the advantage to replace Pt with Pd, a more abundant metal25, extensively used in automotive catalytic converters. Notably, the possible large scale diffusion of highly efficient hydrogen-based fuel cell technology and their application in the transportation technology would markedly reduce the worldwide demand of Pd in automotive converters, leaving open options for other applications, such as HER electrocatalysis. Finally, Pd has the additional value to be nontoxic or allergenic as for other investigated systems such as the Ni-based ones.
Building on these concepts, we prepare a hybrid HER catalyst, f-MWCNTs@Pd/TiO2, based on the combination of functionalized multi-walled carbon nanotubes (f-MWCNTs) and Pd nanoparticles that were mutually integrated within a titanium dioxide (TiO2) shell26. The resulting nanocomposite provides a hierarchical organization of the nanocarbon, metal and metal oxide interfaces, where the specific roles of the individual components are merged to direct HER with an exceptional proficiency. The CNT nanowires enable electron transfer from the electrode to the titania shell, so as to feed the catalytically active Pd nanoparticles. Furthermore, the hydrophilicity of the amorphous TiO2 coating, with its attendant of OH groups, counteracts the CNT hydrophobic nature, and favours water adsorption/dissociation equilibria, and proton translocation steps. In particular, TiO2 defects bordering the MWCNTs surface are expected to play a pivotal role in the HER mechanism. The final outcome is a high efficiency of HER at neutral pH for f-MWCNTs@Pd/TiO2 that reaches, at zero overpotential, a turnover frequency (TOF)0, of 9,460 H2 per hour. This result may set a benchmark for the HER efficiency, as state-of-the art heterogeneous electrocatalysts under similar conditions are an order of magnitude slower. By electrochemical investigations, the catalyst performance is ascribed to an increase in the doping levels of TiO2, together with the generation of surface states at the interface between the semiconductor and the MWCNTs27. Extensive experimental results also demonstrate the presence of cooperative effects within the constituents of f-MWCNTs@Pd/TiO2, whose HER activity greatly outperform that of the isolated components.
Results
Synthesis of the nanohybrids
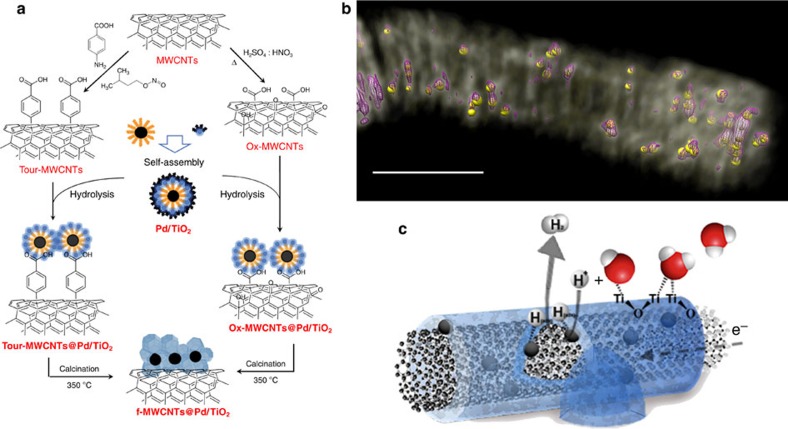
The first step of the synthesis of the MWCNT heterostructures integrating Pd/TiO2 requires the functionalization of the MWCNT surface to promote the metal–oxide adhesion (Fig. 1a). A twofold strategy was adopted to provide the nanocarbon surface with anchor groups, enabling the growth of a co-axial TiO2 outer layer that envelopes the MWCNT throughout its length. The MWCNT were thus treated: (i) under oxidation conditions to yield Ox-MWCNTs@Pd/TiO2 or (ii) by a via diazonium salt radical addition according to the well-known Tour reaction protocol28, yielding Tour-MWCNTs@Pd/TiO2 (Fig. 1a). These functionalized nanoscaffolds provide a direct insight into the role of the chemical-spacer (–OH; –COOH versus benzoic residues) separating the TiO2 shell from the nanocarbon surface, while the final thermal annealing step turns out to seal the MWCNT/TiO2 interface, thus overriding any structural difference, as addressed by the electrocatalytic screening (see below). The co-axial heterostructures self-assemble in solution by a simple one-pot protocol, where the f-MWCNTs are mixed within a Ti(OBu)x phase integrating the Pd nanoparticles (Fig. 1a). Further details on the synthesis can be found in the Supplementary Note 1.
Figure 1. Nanostructured hydrogen-evolving cathode f-MWCNTs@Pd/TiO2.
(a) Schematic representation of the synthetic protocols yielding the f-MWCNTs@Pd/TiO2 electrocatalysts. (b) STEM tomographic reconstruction of f-MWCNTs@Pd/TiO2. Here the region of high density corresponding to the Pd particles is rendered with a violet mesh (raw data). The Pd particles are then localized by placing spherical markers centred in the regions of high density (yellow spheres). Scale bar, 100 nm. (c) General scheme for the HER proposed mechanism by f-MWCNTs@Pd/TiO2 at neutral pH. Water absorption and dissociation occurs at the hydrophilic, porous, TiO2 outer layer (blue shell), promoting the formation of Had (grey) intermediates on the catalytic Pd sites (black).
Structural characterization
Structural and chemisorption evidences confirm that the Pd nanoparticles are deeply embedded into a mesoporous network composed of nanometric TiO2 particles, well connected with each other but leaving accessible the metal phase (Fig. 1b; Supplementary Figs 1 and 2)29. MWCNTs guarantee the electrical conductivity and the efficient delivery of electrons from the electrode to the titania layer. In turn, because of the favourable positions of the titania conduction band and Pd Fermi level, efficient electron transfer from the titania to the Pd occurs, providing the required electrons to the Pd nanoparticles to reduce protons to H2 (Fig. 1c). The process is facilitated by the intimate three-dimensional contact between the Pd nanoparticles and TiO2 that maximize the metal support interface.
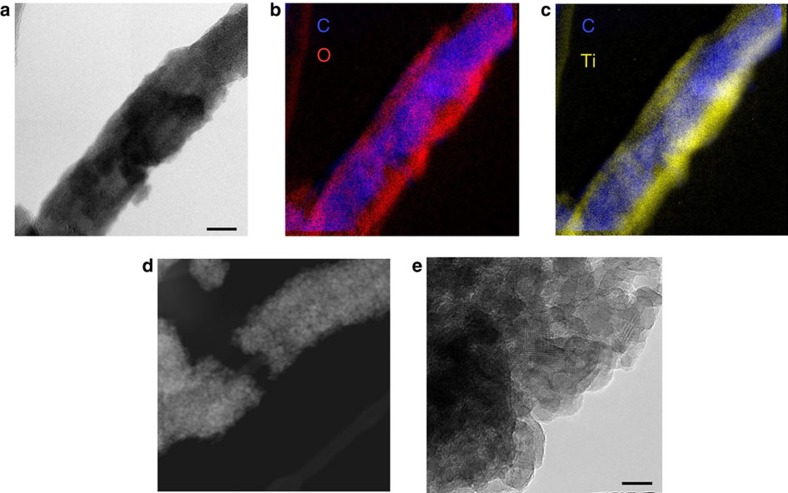
Figure 2a–c shows representative transmission electron microscopy (TEM) images of the nanoarchitectures, where a uniform titania layer surrounding the f-MWCNTs is clearly observed. The starting Pd nanoparticles with a size of 1.8±0.3 nm are embedded within the titania layers. X-ray diffraction, X-ray photoemission spectroscopy and TEM characterization of the final hybrid materials26, and comparison with that of the Pd/TiO2 and of the starting protected Pd nanoparticles confirm the formation of homogeneous hierarchical materials30. It is important to note that for both Ox-MWCNTs@Pd/TiO2 and Tour-MWCNTs@Pd/TiO2, the coverage with the titania layer is not complete, but zones of bare MWCNTs are clearly observed (Fig. 2d). This is an advantage as it provides the hybrid material with points of direct contact between the nanotubes and the electrode, securing an improved electric conductivity within the nanocomposite and an efficient electron transfer to the inorganic phase.
Figure 2. Characterization of MWCNTs@Pd/TiO2 nanohybrids.
(a) Representative TEM image of the f-MWCNTs@Pd/TiO2 nanoarchitectures prepared by self-assembly, where f-MWCNTs are embedded inside a layer of TiO2 that contains Pd nanoparticles dispersed within. Scale bar, 20 nm. (b,c) Energy filtered TEM images highlighting the elemental distribution of the TiO2 shell (red and yellow for O and Ti atoms, respectively) surrounding the f-MWCNT backbone (C atoms in blue). (d) STEM-HAADF of a typical Ox-MWCNT@Pd/TiO2. (e) Large magnification HRTEM of an area of a thermally annealed Ox-MWCNT@Pd/TiO2 showing the formation of crystalline TiO2 anatase phase (scale bar, 10 nm).
In the as-prepared hybrids Ox-MWCNTs@Pd/TiO2 and Tour-MWCNTs@Pd/TiO2, the titania layer is in its amorphous phase, as we failed to find any TiO2 crystal grains by high-resolution TEM (HRTEM). Raman analyses of the sample confirmed that the titania layer is not crystalline, as no peaks for distinctive crystal phases were found in any area. The integration of the amorphous TiO2 was however confirmed by Fourier transform infrared, where together with the signature C=C stretching at 1,625 cm−1 of the CNTs, we observed appearance of the characteristic Ti–O–Ti vibration modes at 700 and 672 cm−1 after reaction with the inorganic Pd/TiO2 (Supplementary Figs 3 and 4). The amorphous state of the TiO2 may have important implications in enhancing the affinity for water molecules, thanks to the presence of Ti–OH groups. On the other hand, as revealed by HRTEM, the thermal annealing treatment causes a transition to the anatase phase with crystallites size that averages 10 nm (Fig. 2e; Supplementary Fig. 5). The annealing also removes all the organic groups from the material, tightening the contact between the MWCNTs and the TiO2, which is crucial to enhance the catalytic performance as a result of the increased level of heterojunctions (Supplementary Fig. 6). Because in the calcined samples the organic spacers are now removed, we expect that the structural differences between the Ox-MWCNTs and Tour-MWCNTs nanscaffolds are no longer present, so that they are likely to converge towards an analogous HER material as it has been confirmed by the electrocatalytic studies (see discussion below).
From the tomographic reconstruction (Fig. 1b), the Pd nanoparticles are also evident, as resulting from the higher contrast in the scanning transmission electron microscopy (STEM) projections due to the high atomic number of Pd with respect to the other elements. However, due to the experimental missing wedge in sample rotation during acquisition (sample tilt −60°/+60°), they appear slightly elongated in the direction perpendicular to the wire axis in the reconstruction.
Electrochemical properties
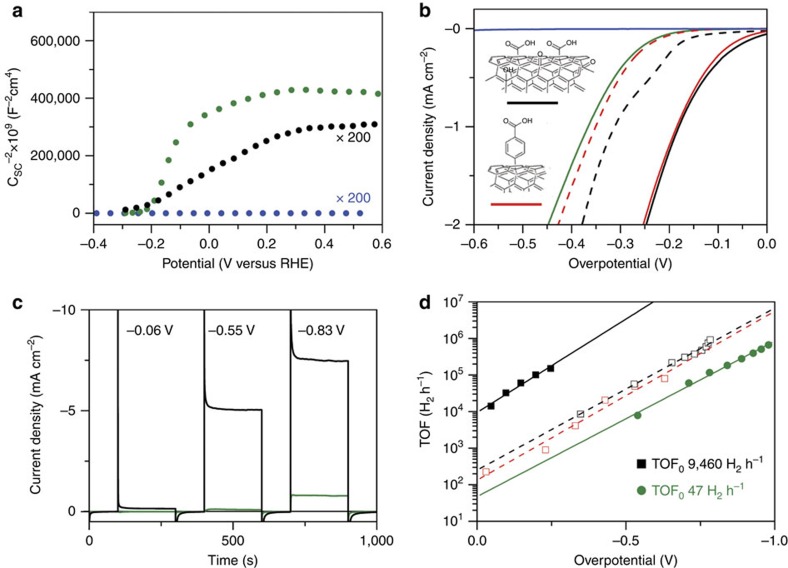
The electrochemical investigation of the annealed nanohybrids, f-MWCNTs@Pd/TiO2, evidenced remarkable changes in the electronic structure of TiO2 caused by its integration with MWCNTs, changes that will in turn favourably contribute to the enhancement of the electrocatalytic properties of the hierarchical Pd/TiO2 structure. An increase of (i) the number of free electrons in the titania conduction band and (ii) surface defect density were in fact highlighted by the analysis of the interfacial capacity (Csc) of f-MWCNTs@Pd/TiO2 compared with pristine Pd/TiO2. In Fig. 3a, the Csc values, measured by electrochemical impedance spectroscopy, are plotted according to the classical Mott–Schottky (M–S) equation31:
Figure 3. Electrocatalysis of HER on nanohybrid cathodes.
Films were prepared by drop-casting a controlled amount of the catalyst inks onto either carbon screen-printed electrode (SPE) or glassy carbon (GC) electrodes (both substrates are catalytically inert towards the HER process). (a) Mott–Schottky plots (C−2 versus potential) for the various nanostructured systems in phosphate buffer, pH=7.4, obtained at a fixed frequency of 1 kHz: f-MWCNT@Pd/TiO2 (black circle), Pd/TiO2 (green circle) and the pristine electrode surface (blue circle). (b) Linear sweep voltammetry and (c) chronoamperometric curves in phosphate buffer (pH 7.4) for the pristine SPE electrode (blue line), after the deposition of the nanocomposites Ox-MWCNT@Pd/TiO2 (dashed black line), Tour-MWCNT@Pd/TiO2 (dashed red line), Pd/TiO2 (green line) and calcined f-MWCNT@Pd/TiO2 (solid black and red lines) Scan rate=2 mV s−1. (d) Electrocatalytic performances for Ox-MWCNT@Pd/TiO2 (dashed black line), Tour-MWCNT@Pd/TiO2 (dashed red line), Pd/TiO2 (green line) and calcined f-MWCNT@Pd/TiO2 (solid black line): TOF as a function of η. η=Eapp−E0—iss × Rs, where Eapp is the applied potential, E0 is the thermodynamic potential corrected for the pH (E0 [H+/H2]=−0.21–0.059 × pH=−0.65 V versus Ag/AgCl and iss × RS is the correction for the ohmic drop (iss is the stationary state current measured during the potential step and RS is the uncompensated resistance). TOF0 is the turnover frequency at zero overpotential (TOF at η=0 V). All potentials are reported versus RHE.
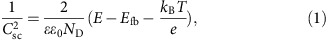 |
where ɛ is the relative dielectric constant, ɛ0 the permittivity of vacuum, e the electronic charge, kB the Boltzmann constant. From the analysis of the narrow E-dependent linear region (E∼0.00 V), a flat-band potential Efb=−0.22 V was obtained for all systems, in good agreement with the reported values for TiO2 at neutral pH32. However, on passing from Pd/TiO2 to f-MWCNT@Pd/TiO2, the apparent doping density (ND), and hence the amount of free electrons in the TiO2 conduction band, increased by ∼600 times and it further depends on the f-MWCNT concentration (Supplementary Figs 7 and 8; Supplementary Table 1)33. The larger availability of free electrons at the MWCNT/TiO2 interface is expected to play a favourable effect on the HER kinetics, especially at low overpotentials.
Finally, the analysis of the M–S plots at more positive potentials, where the capacity attains a constant value, provided evidence for the presence of extended surface states, whose density (proportional to Csc) largely increased on passing from Pd/TiO2 to f-MWCNTs@Pd/TiO2 (Supplementary Table 1)34. Such defects on the surface of the titania nanocrystals (oxygen vacancies and incompletely coordinated Ti atoms)35, that would be mostly localized at the interface with MWCNTs, may provide efficient catalytic sites for the adsorption and catalytic dissociation of water molecules, an important step for the promotion of HER in neutral and alkaline media (Had formation). As a matter of fact, as the kinetic studies of f-MWCNTs@Pd/TiO2 show (Tafel slope analysis and [H+] dependence; Supplementary Figs 9–11), water dissociation and adsorbed hydrogen formation would represent the rate-determining step of the HER in the present conditions.
Electrocatalytic performance of HER
The HER activity of the nanohybrids was investigated by linear sweep voltammetry and chronoamperometry (CA), in de-aerated phosphate buffer solutions at pH 7.4 (Fig. 3b,c), using a standard three-electrode electrochemical cell, equipped with an Ag/AgCl (3 M) reference electrode and a platinum counter electrode. The hydrogen-evolving activity of the catalyst was assessed qualitatively and quantitatively using a scanning electrochemical microscope (SECM)36,37. The molecular hydrogen probe, namely, a platinum ultramicrometric tip biased at 0.4 V (versus RHE), was located at few micrometres from the electrode surface, under conditions of constant hydrogen collection efficiency (Supplementary Fig. 12). The SECM screening method was used to probe a few mm2 surface providing a faster and more direct way to quantify the electrocatalytic efficiency of the reactive material with respect to standard (colorimetric and chromatographic) methods.
The catalytic onset for the HER of both Ox-MWCNTs@Pd/TiO2 and Tour-MWCNTs@Pd/TiO2 electrodes is located at E<−0.2 V, while the overpotentials measured at an arbitrary cathodic current threshold of 1 mA cm−2 (corresponding to a hydrogen evolution rate of 19 μmol h−1 cm−2) were −0.31 and −0.35 V, respectively (Fig. 3b). We attribute such differences in the overpotentials to the organic spacer between the two nanocarbons and the titania. Namely, the benzoic groups separate the titania layer from the nanotubes more than simple carboxylic or hydroxyl groups, leading to a less efficient electronic communication. The optimized interface was obtained by removing the organic functionalization with a thermic treatment. As previously mentioned, calcination at 350 °C for 3 h increase the interaction between the MWCNT and TiO2, resulting in significantly higher HER current and higher apparent doping density (see Fig. 3b the comparison between dashed and solid lines; Supplementary Fig. 8; Supplementary Table 1). After calcination, Ox-MWCNTs@Pd/TiO2 and Tour-MWCNTs@Pd/TiO2 merge to a structure with the same catalytic property: f-MWCNTs@Pd/TiO2. A general remarkable improvement in the HER performance was observed, as evidenced by the positive shift of η (at 1 mA cm−2) to a value of −0.17 V (vis-à-vis to −0.37 V in the case of Pd/TiO2). Notice that, under the above conditions, protons are reduced on massive platinum electrodes at about −0.10 V and that at this pH about 50% of the overpotential is due to the concentration overpotential38,39. The Tafel slopes of ∼130 mV per decade (Supplementary Fig. 9; Supplementary Table 1) were in good agreement with literature values for HER at Pd electrodes40, and, combined with the unitary slope observed in the log(i)/log[H+] plot (experiments in non-aqueous media, Supplementary Figs 10 and 11), emphasize the role of the Volmer step (water dissociation and formation of reactive intermediate Had: H2O+Pd+e=Pd−Had+OH−) as the rate-determining step41. The availability of free protons in neutral and alkaline media is critical and, in such a scenario, the titania shells would promote the adsorption of water molecule and its dissociative adsorption at the boundaries with the Pd clusters, as depicted schematically in Fig. 1c. A similar mechanism was recently reported in bifunctional Pt-Ni(OH)2 nanoystems to explain their efficient promotion of electrocatalytic HER in alkaline media8. It can also be inferred that the observed increase of titania surface defects, associated to MWCNTs, would enhance the dissociative adsorption process, thus explaining the increased performance of f-MWCNTs@Pd/TiO2 nanohybrid systems.
TOF quantification
Quantification of electrocatalytic activity was performed by the combination of different electrochemical techniques. The current transients measured in the CA experiments (Fig. 3c), in which the cathode potential was stepped back and forth between 0 V and a negative value in the range −0.5/−1.0 V, were stable and reproducible. Importantly, all catalysts displayed great chemical and mechanical stability (even under massive hydrogen evolution conditions) without any significant activity loss after repetitive cycling and after >45 h operation (<15% activity loss; Supplementary Fig. 13). The TOF at each overpotential η, that is, the moles of hydrogen generated by the electroactive Pd catalytic sites in the time unit, was then derived as described in equation (2) from the integrated currents at the various substrates (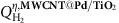 ) after subtraction of the background contribution (
) after subtraction of the background contribution (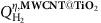 , measured in the absence of Pd nanoparticles) and after normalization by the amount of electroactive Pd (QPd; Supplementary Fig. 14).
, measured in the absence of Pd nanoparticles) and after normalization by the amount of electroactive Pd (QPd; Supplementary Fig. 14).
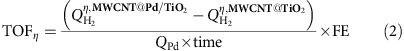 |
The faradaic efficiency (FE) term in equation (2), that is, the fraction of cathodic current that actually generates hydrogen, was determined in situ during electrolysis using the SECM-Pt hydrogen probe (Supplementary Fig. 15). A preliminary determination of the collection efficiency of the probe was obtained following a reported procedure42, and thoroughly described in Supplementary Notes 2 and 4 (Supplementary Figs 16 and 17). In Fig. 3d, the TOF values for the various nanohybrids are finally reported considering a FE of 90% (Supplementary Notes 3 and 4): the Tafel-like behaviour demonstrates the good stability of the catalytic film and a well-behaved ohmic contact to the underlying conducting substrate. The electrocatalytic performance of the optimized f-MWCNTs@Pd/TiO2 systems resulted in an outstanding TOF of 15000 H2 per hour at η=50 mV.
Discussion
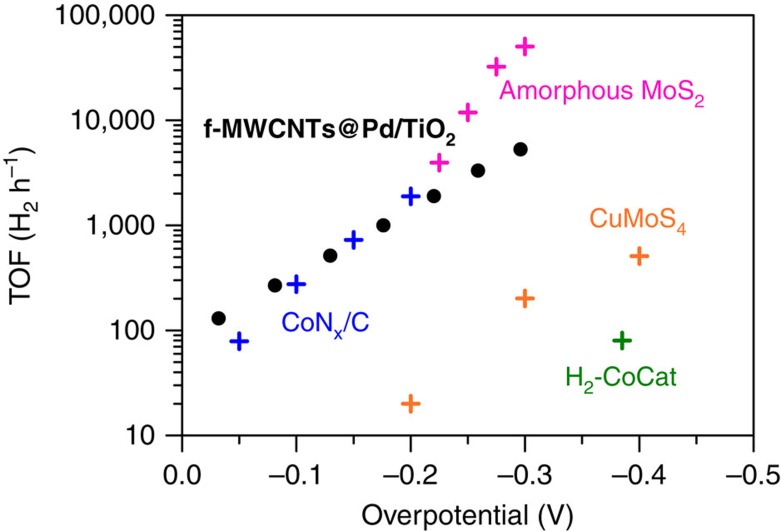
Hydrogen is considered one of the viable alternatives to fossil fuels. However, its sustainable production from water splitting is still hampered by major thermodynamic and kinetic hurdles of electrocatalysis and energy materials. In fact, despite the huge research efforts in this area, H2 production by water electrolysis occurs with prohibitive overpotentials and requires >1.8 V applied voltage, together with harsh strong alkaline or acidic pH conditions. The unsolved problem of the HER calls for the engineering of Pt-free catalyst that can work with low overpotential and remarkable activity, in unfavourable conditions of H+ concentration, that is, at neutral pH. In this scenario, our results provide an important step forward in the design of electrocatalytic nanomaterials for HER. A direct comparison with state-of-the-art heterogeneous HER electrocatalysts that operate at neutral pH (Fig. 4) ranks the thermal annealed nanohybrids f-MWCNTs@Pd/TiO2 among the best performing materials so far reported (see Supplementary Table 2 for an extensive comparison of TOF, exchange current density and Tafel slope values) and demonstrates the ability to operate at a very high rate at low overpotential.
Figure 4. Comparison of mass-normalized TOF with other catalysts.
Direct comparison of TOF plots for f-MWCNTs@Pd/TiO2 nanohybrid together with other recently reported heterogeneous HER electrocatalysts that operate at neutral pH. The TOF herein reported are calculated considering the entire catalyst loading on the various electrodes and using rotating disk glassy carbon electrode (rotation speed of 1,600 r.p.m.). The data are adapted from: ref. 47 for CoNx/C (2015), ref. 48 for amorphous MoS2 (2011), ref. 49 for H2-CoCat (2012) and ref. 50 for CuMoS4 (2012).
Furthermore, extrapolation of the TOF-η semilogaritmic plot to zero overpotential provided the TOF0 intercept value, which is an optimal descriptor of the intrinsic performance of electro-catalysts43. For f-MWCNTs@Pd/TiO2, the TOF0 was 9,460 H2 per hour, whereas in the case of Pd/TiO2, a significantly lower TOF0 value of 47 H2 per hour was measured, thus confirming the important role played by the carbon nanomaterials in promoting the catalytic activity of the metal nanoparticles. By changing the doping level and surface state density of the titania layers through hybridization with MWCNTs, a marked enhancement of the electrocatalytic properties of the nanocomposites is observed, as it is evident from the comparison between catalysts with different MWCNTs concentrations (Supplementary Fig. 18). In this respect, films obtained by physically mixing Ox-MWCNT with Pd/TiO2 and Ox-MWCNT with Pd/TiO2 or the composite f-MWCNTs@Pd (Supplementary Fig. 19) gave significantly higher capacitive currents but much worse electrocatalytic activities and lower stability (Supplementary Figs 20 and 21). This highlights the synergistic effects and the advantages derived from the nanoscale integration of the various components in the f-MWCNTs@Pd/TiO2 nanohybrids. Moreover, the 50 times higher TOF0 values of f-MWCNTs@Pd/TiO2 with respect to Ox-MWCNTs@Pd/TiO2 and Tour-MWCNTs@Pd/TiO2 highlight again the essential role of the MWCNT/TiO2 interface to promote an efficient HER process.
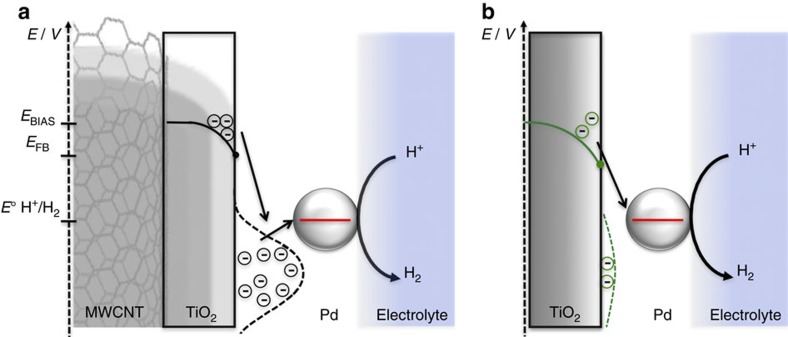
As schematically depicted in Fig. 5, MWCNTs would therefore promote the electrocatalytic activity of the Pd/TiO2 by two concurrent electronic effects: (i) since the flat-band potential of TiO2 is only 220 mV more negative than the potential of the hydrogen electrode32, the amount of free electrons at the titania surface that may promote the HER at low overpotentials is rather small. In such conditions, the increased doping level induced by MWCNTs (Supplementary Table 1) is going to exert a strongly positive effect on the HER kinetics. In addition, (ii) the presence of extensive surface states at the TiO2/MWCNTs interface would promote a better coupling with the Pd electronic levels, globally resulting in faster kinetics of the HER process.
Figure 5. Schematic energy level representation.
Band-bending diagram and schematic representation of the energy levels involved in the HER process. The scheme also depicts qualitatively the surface states distributions: (a) f-MWCNTs@Pd/TiO2 and (b) Pd/TiO2 (green dashed line). EBIAS: −0.35 V; Efb: −0.22 V; E° [H+/H2]: 0 V (versus RHE).
In particular, we have designed a three-component interface, where MWCNT template the one-dimensional morphology of a Pd/TiO2 units active layer. The catalyst composition and morphology are shaped to leverage the key steps of the HER mechanism in neutral pH conditions, namely: (i) H2O binding and electron-induced dissociation at the MWCNT@TiO2 metal oxide surface; (ii) stabilization of the atomic hydrogen intermediate on Pd; and (iii) hydrogen evolution by reaction of the adsorbed atomic hydrogen at the Pd/TiO2 interface. This functional synergy stems from a favourable interplay of kinetic and thermodynamic factors, yielding an efficient Pt-free catalyst package with (i) notable performances in neutral water media (TOF0 of 9,460 H2 per hour, negligible onset potential for HER, exchange current density of 6 × 10−5 A cm−2 ), (ii) excellent robustness (current decay <15% after 45 h electrolysis) and (iii) strong correlation between HER activity and the electronic characteristics of the MWCNT/TiO2 interface (50 times faster HER after optimization via thermal annealing).
To summarize, we have presented evidence on how the hierarchical integration of multi-walled CNTs and metal/metal oxides within a single nanostructure can leverage the potentiality of HER electrocatalysis at neutral pH. Moreover, the modularity of the catalyst fabrication is open to a straightforward fine-tuning of the metal–metal oxide pairs and nanoscaffolds to meet the HER requirements spanning a wide range of experimental conditions. This approach is expected to provide insights in the HER electrocatalytic mechanisms while implementing versatility and flexibility of the catalyst package for a sustainable hydrogen economy.
Methods
Chemicals
All glassware was dried in an oven set to a temperature of 80 °C for 24 h before use. All reagents were purchased from Sigma Aldrich and used without further purification. Phosphate buffer (PB) electrolytes were prepared readily before experiments from reagent-grade chemicals, mixing appropriate amount of NaH2PO4 and Na2HPO4 salts.
Electrochemistry
Linear sweep voltammetry, cyclic voltammetry (CV), chronoamperometry (CA) and electrochemical impedance spectroscopy experiments were carried out in a three-electrode electrochemical cell using as working electrodes screen-printed electrodes (supplied by DROPSENS S.L., model DS110 diameter 4 mm), glassy carbon electrodes (CH Instruments, diameter 3 mm) or on indium tin oxide (purchased from Kuramoto Seisakusho Co., Ltd, Tokyo, Japan) as a working electrode. A Biologic SP300 potentiostat was used as the work station for all the electrochemical experiments. Thin films of the different nanocomposites were prepared on the substrate working electrodes by deposition of the respective catalyst inks (methanol suspensions, 1.6 mg ml−1). The best performing films (Supplementary Fig. 22) combine good conductivity and an optimum catalyst loading (170 μg cm−2). Thin compact films were obtained with 5 μl aliquots subsequently added until a total deposited volume of 100 μl was reached. Those were then allowed to dry in the dark for at least 12 h and the film thickness on the electrode surface, measured with a Tencor AlphaStep profilometer, was 10±3 μm with a roughness of 3±1 μm.
Transmission electron microscopy
HRTEM and STEM in high-angle annular dark-field mode (HAADF) were performed on a JEOL JEM-2200FS microscope, working at 200 kV. Energy filtered maps were obtaining using an in-column energy spectrometer (Ω-type). In the STEM-HAADF images (as for the tomography projections), the camera length value was set to assure an inner cutoff angle of the STEM detector >100 mrad, to reduce the contribution from coherent contrast to the minimum and to enhance the contrast of Pd particles with respect to TiO2 (higher atomic number of Pd with respect to Ti). The three-dimensional tomography was obtained by a simultaneous iterative reconstruction technique (SIRT) reconstruction (20 iterations) of a series of 46 projections acquired in STEM-HAADF (from −30 to +60° with 2° step).
Experiments in non-aqueous media
Electrochemical experiments were carried out in an airtight single-compartment cell described elsewhere, using platinum and silver spirals, respectively, as counter and quasi-reference electrodes44. The E1/2 values, reported in the Ag/AgCl/KCl (3 M) scale, have been determined by adding decamethylferrocene as an internal standard at the end of each experiment. All the experiments were conducted in anhydrous acetonitrile and drying the supporting electrolyte, tetrabutylammonium hexafluorophosphate TBAPF6, for 60 h under vacuum before each experiments. Additions of [DMFH](OTf):DMF (1 mol:1mol) were made from a solution with a [DMFH]+ concentration of 4.1 M, following a procedure previously described24. All the solutions were argon-saturated for 30 min before performing the experiments.
Experiments in aqueous media
Electrochemical experiments were carried out in a home-made cell having a 5 mm diameter opening in front of the substrate electrodes. An O-ring ensures a perfect tightening of the assembly thanks to connecting screws that fit directly into the body of the cell. All the experiments were carried out in phosphate buffer 0.1 M (pH=7.4) freshly prepared readily before each experiment. The electrochemical cell was equipped with a platinum spiral counter electrode and a Ag/AgCl/KCl (3 M) reference electrode. Potentials were then reported in the reversible hydrogen electrode scale by shifting the potentials by (ERHE=EAg/AgCl+0.436 V). All the solutions were thoroughly degassed with argon for at least 25 min before each experiment. Comparison of TOF performances for f-MWCNTs@Pd/TiO2 and other state-of-the-art HER electrocatalysts was done in a rotating disk electrode (glassy carbon) configuration (Fig. 4). CA measurements have been performed at a rotation speed of 1,600 r.p.m. and under continuous Ar flow. For a straightforward comparison of the catalysts, mass-normalized TOF data were obtained considering the entire loading of Pd on the electrode (1 wt.%) and not on an electroactive area basis.
In the M–S analysis, we scanned the frequency from 1 kHz to 1 Hz (three frequencies per decade) and we varied the potential in the range from 0.8 to −0.4 V (versus RHE). All the experiments were conducted in a phosphate buffer solution at pH=7.4. The following constants were used for the M–S data processing: kB=1.38 × 10−23 J K−1, T=298 K, e=1.6 × 10−19 C, ɛ0=8.86 × 10−12 F m−1, ɛ=48 F m−1 for anatase TiO2 (ref. 45).
For the SECM experiments, a CH Instruments CHI 910B SECM apparatus was used. A 15 μm platinum ultramicroelectrode (CHI Instruments, USA) was used as the tip working electrode. The approaching curves to the substrates were obtained via feedback mode in a classic three-electrode cell, using 1 mM ferrocenemethanol in a 100 mM LiClO4 aqueous solution. The surface generation-tip collection experiments were carried out in argon-saturated solution (pH 7.4)46.
Data availability
The data that support the findings of this study are available from the corresponding authors on request.
Additional information
How to cite this article: Valenti, G. et al. Co-axial heterostructures integrating palladium/titanium dioxide with carbon nanotubes for efficient electrocatalytic hydrogen evolution. Nat. Commun. 7, 13549 doi: 10.1038/ncomms13549 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Supplementary Figures 1-22, Supplementary Tables 1-2, Supplementary Notes 1-4 and Supplementary References
Acknowledgments
This work is supported by the Italian Ministero dell'Istruzione, Università e Ricerca (FIRB RBAP11C58Y, PRIN-2010N3T9M4, PRIN-2009Z9ASCA and FIRB RBAP11-ETKA_006), Universities of Bologna and Trieste, INSTM, the Seventh Framework Programme [FP7/2007–2013] under grant agreement no. 310651 (SACS project), EU Cost action CM1104.
Footnotes
Author contributions G.V. and A.B. contributed equally to this work. M.Me. and M.C. performed the synthetic tasks; L.N. and G.B. performed the HRTEM and STEM analysis; G.V. and A.B. optimized the deposition protocols, performed and analysed the electrochemical and electrocatalytic experiments; G.V., A.B. and M.Ma., performed the electrochemistry investigation in non-aqueous media; G.V., A.B. and S.R. performed the scanning electrochemical experiments; R.J.G., M.B., M.P., P.F. and F.P. planned and supervised the research and co-wrote the paper with contributions from other authors. All authors discussed the results and commented the manuscript.
References
- Balzani V. & Armaroli N. The hydrogen issue. ChemSusChem 4, 21–36 (2011). [DOI] [PubMed] [Google Scholar]
- Callejas J. et al. Electrocatalytic and photocatalytic hydrogen production from acidic and neutral-pH aqueous solutions using iron phosphide nanoparticles. ACS Nano 8, 11101–11107 (2014). [DOI] [PubMed] [Google Scholar]
- Zhang B., Wen Z., Ci S., Chen J. & He Z. Nitrogen-doped activated carbon as a metal free catalyst for hydrogen production in microbial electrolysis cells. RSC Adv. 4, 49161–49164 (2014). [Google Scholar]
- Shiraishi Y., Sugano Y., Tanaka S. & Hirai One-Pot, T. synthesis of benzimidazoles by simultaneous photocatalytic and catalytic reactions on Pt@TiO2 nanoparticles. Angew. Chem. Int. Ed. 49, 1656–1660 (2010). [DOI] [PubMed] [Google Scholar]
- Markovic N. M., Grgur B. N. & Ross P. N. Temperature-dependent hydrogen electrochemistry on platinum low-index single-crystal surfaces in acid solutions. J. Phys. Chem. B 101, 5405–5413 (1997). [Google Scholar]
- Sheng W., Gasteiger H. A. & Shao-Horn Y. Hydrogen oxidation and evolution reaction kinetics on platinum: acid vs alkaline electrolytes. J. Electrochem. Soc. 157, B1529–B1536 (2010). [Google Scholar]
- Mazzaro R. et al. Uniform functionalization of high-quality graphene with platinum nanoparticles for electrocatalytic water reduction. ChemistryOpen 4, 268–273 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaraman R. et al. Enhancing hydrogen evolution activity in water splitting by tailoring Li+-Ni(OH)2-Pt interfaces. Science 334, 1256–1260 (2011). [DOI] [PubMed] [Google Scholar]
- Paracchino A., Laporte V., Sivula K., Graetzel M. & Thimsen E. Highly active oxide photocathode for photoelectrochemical water reduction. Nat. Mater. 10, 456–461 (2011). [DOI] [PubMed] [Google Scholar]
- Li Y. et al. MoS2 nanoparticles grown on graphene: an advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 133, 7296–7299 (2011). [DOI] [PubMed] [Google Scholar]
- Kibsgaard J., Jaramillo T. F. & Besenbacher F. Building an appropriate active-site motif into a hydrogen-evolution catalyst with thiomolybdate [Mo3S13]2- clusters. Nat. Chem. 6, 248–253 (2014). [DOI] [PubMed] [Google Scholar]
- Chen W.-F., Muckerman J. T. & Fujita E. Recent developments in transition metal carbides and nitrides as hydrogen evolution electrocatalysts. Chem. Commun. 49, 8896–8906 (2013). [DOI] [PubMed] [Google Scholar]
- Cao B., Veith G. M., Neuefeind J. C., Adzic R. R. & Khalifah P. G. Mixed close-packed cobalt molybdenum nitrides as non-noble metal electrocatalysts for the hydrogen evolution reaction. J. Am. Chem. Soc. 135, 19186–19192 (2013). [DOI] [PubMed] [Google Scholar]
- Popczun E. J. et al. Nanostructured nickel phosphide as an electrocatalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 135, 9267–9270 (2013). [DOI] [PubMed] [Google Scholar]
- Popczun E. J., Read C. G., Roske C. W., Lewis N. S. & Schaak R. E. Highly active electrocatalysis of the hydrogen evolution reaction by cobalt phosphide nanoparticles. Angew. Chem. Int. Ed. 53, 5427–5430 (2014). [DOI] [PubMed] [Google Scholar]
- Tian J., Liu Q., Asiri A. M. & Sun X. Self-supported nanoporous cobalt phosphide nanowire arrays: an efficient 3D hydrogen-evolving cathode over the wide range of pH 0–14. J. Am. Chem. Soc. 136, 7587–7590 (2014). [DOI] [PubMed] [Google Scholar]
- Maeda K. et al. Photocatalytic activities of graphitic carbon nitride powder for water reduction and oxidation under visible light. J. Phys. Chem. C 113, 4940–4947 (2009). [Google Scholar]
- Subbaraman R. et al. Trends in activity for the water electrolyser reactions on 3d M(Ni,Co,Fe,Mn) hydr(oxy)oxide catalysts. Nat. Mater. 11, 550–557 (2012). [DOI] [PubMed] [Google Scholar]
- Zhang Y. & Angelidaki I. Microbial electrolysis cells turning to be versatile technology: recent advances and future challenges. Water Res. 56, 11–25 (2014). [DOI] [PubMed] [Google Scholar]
- Symes M. D. & Cronin L. Decoupling hydrogen and oxygen evolution during electrolytic water splitting using an electron-coupled-proton buffer. Nat. Chem. 5, 403–409 (2013). [DOI] [PubMed] [Google Scholar]
- Cargnello M. et al. Control of metal nanocrystal size reveals metal-support interface role for ceria catalysts. Science 341, 771–773 (2013). [DOI] [PubMed] [Google Scholar]
- Toma F. M. et al. Efficient water oxidation at carbon nanotube-polyoxometalate electrocatalytic interfaces. Nat. Chem. 2, 826–831 (2010). [DOI] [PubMed] [Google Scholar]
- Tasis D., Tagmatarchis N., Bianco A. & Prato M. Chemistry of carbon nanotubes. Chem. Rev. 106, 1105–1136 (2006). [DOI] [PubMed] [Google Scholar]
- Le Goff A. et al. From hydrogenases to noble metal–free catalytic nanomaterials for H2 production and uptake. Science 326, 1384–1387 (2009). [DOI] [PubMed] [Google Scholar]
- Greenwald N. N. & Earnshaw A. Chemistry of the Elements Pergamon Press (1985). [Google Scholar]
- Cargnello M. et al. Multiwalled carbon nanotubes drive the activity of metal@oxide core–shell catalysts in modular nanocomposites. J. Am. Chem. Soc. 134, 11760–11766 (2012). [DOI] [PubMed] [Google Scholar]
- Tomkiewicz M. The potential distribution at the titanium dioxide aqueous electrolyte interface. J. Electrochem. Soc. 126, 1505–1510 (1979). [Google Scholar]
- Price B. K. & Tour J. M. Functionalization of single-walled carbon nanotubes ‘on water'. J. Am. Chem. Soc. 128, 12899–12904 (2006). [DOI] [PubMed] [Google Scholar]
- Bakhmutsky K. et al. A versatile route to core–shell catalysts: synthesis of dispersible M@Oxide (M=Pd, Pt; Oxide=TiO2, ZrO2) nanostructures by self-assembly. ChemSusChem 5, 140–148 (2012). [DOI] [PubMed] [Google Scholar]
- Cargnello M. et al. A versatile approach to the synthesis of functionalized thiol-protected palladium nanoparticles. Chem. Mater. 23, 3961–3969 (2011). [Google Scholar]
- Bard A. J. & Faulkner L. R. Electrochemical Methods. Fundamentals and Applications John Wiley & Sons (2001). [Google Scholar]
- Kavan L., Grätzel M., Gilbert S. E., Klemenz C. & Scheel H. J. Electrochemical and photoelectrochemical investigation of single-crystal anatase. J. Am. Chem. Soc. 116, 6716–6723 (1996). [Google Scholar]
- Zhang W.-D., Jiang L.-C. & Ye J.-S. Photoelectrochemical study on charge transfer properties of ZnO nanowires promoted by carbon nanotubes. J. Phys. Chem. C. 113, 16247–16253 (2009). [Google Scholar]
- Sato N. Electrochemistry at Metal and Semiconductor Electrodes Elsevier Science B. V. (1998). [Google Scholar]
- Diebold U. The surface science of titanium dioxide. Surf. Sci. Rep. 48, 53–229 (2003). [Google Scholar]
- Wittstock G., Burchardt M., Pust S. E., Shen Y. & Zhao C. Scanning electrochemical microscopy for direct imaging of reaction rates. Angew. Chem. Int. Ed. 46, 1584–1617 (2007). [DOI] [PubMed] [Google Scholar]
- Bard A. J. & Mirkin M. V. Scanning Electrochemical Microscopy Marcel Dekker Inc. (2001). [Google Scholar]
- Chen L. et al. A super-efficient cobalt catalyst for electrochemical hydrogen production from neutral water with 80 mV overpotential. Energy Environ. Sci. 7, 329–334 (2014). [Google Scholar]
- Shinagawa T. & Takanabe K. Electrocatalytic hydrogen evolution under densily buffered neutral pH condition. J. Phys. Chem. C 119, 20453–20458 (2015). [Google Scholar]
- Conway B. E. & Tilak B. V. Interfacial processes involving electrocatalytic evolution and oxidation of H-2, and the role of chemisorbed H. Electrochim. Acta 47, 3571–3594 (2002). [Google Scholar]
- Durst J. et al. New insights into the electrochemical hydrogen oxidation and evolution reaction mechanism. Energy Environ. Sci. 7, 2255–2260 (2014). [Google Scholar]
- Sanchez-Sanchez M. C., Rodriguez-Lopez J. & Bard A. J. Scanning electrochemical microscopy. 60. Quantitative calibration of the SECM substrate generation/tip collection mode and its use for the study of the oxygen reduction mechanism. Anal. Chem. 80, 3254–3260 (2008). [DOI] [PubMed] [Google Scholar]
- Costentin C., Drouet S., Robert M. & Saveant J.-M. Turnover numbers, turnover frequencies, and overpotential in molecular catalysis of electrochemical reactions. cyclic voltammetry and preparative-scale electrolysis. J. Am. Chem. Soc. 134, 11235–11242 (2012). [DOI] [PubMed] [Google Scholar]
- Scarel F. et al. A molecular shuttle driven by fullerene radical-anion recognition. Chem. Eur. J 18, 14063–14068 (2012). [DOI] [PubMed] [Google Scholar]
- Ye M., Gong J., Lai Y., Lin C. Z. & Lin Z. High-efficiency photoelectrocatalytic hydrogen generation enabled by palladium quantum dots-sensitized TiO2 nanotube arrays. J. Am. Chem. Soc. 134, 15720–15723 (2012). [DOI] [PubMed] [Google Scholar]
- Valenti G. et al. Creation of reactive micro patterns on silicon by scanning electrochemical microscopy. J. Phys. Chem. C 114, 22165–22170 (2010). [Google Scholar]
- Liang H.-W. et al. Molecular metal–Nx centres in porous carbon for electrocatalytic hydrogen evolution. Nat. Commun. 6, 7992 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merki D., Fierro S., Vrubel H. & Hu X. Amorphous molybdenum sulfide films as catalysts for electrochemical hydrogen production in water. Chem. Sci. 2, 1262–1267 (2011). [Google Scholar]
- Cobo S. et al. A Janus cobalt-based catalytic material for electro-splitting of water. Nat. Mater. 11, 802–807 (2012). [DOI] [PubMed] [Google Scholar]
- Tran P. D. et al. Copper molybdenum sulfide: a new efficient electrocatalyst for hydrogen production from water. Energy Environ. Sci. 5, 8912–8916 (2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-22, Supplementary Tables 1-2, Supplementary Notes 1-4 and Supplementary References
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors on request.