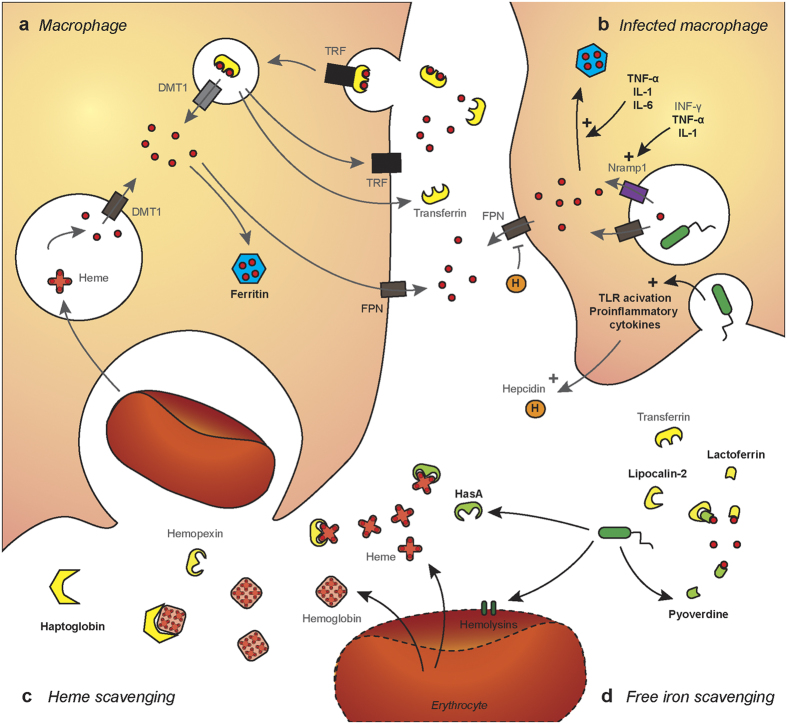
Figure 5. Iron scavenging and regulation in vivo.
(a) Macrophages acquire iron via transferrin receptor (TRF)-mediated endocytosis of transferrin or via recycling of senescent erythrocytes. Macrophages degrade heme using heme oxygenase to generate iron, biliverdin and carbon monoxide. Iron is then transferred to the cytoplasm by DMT1 and either stored using ferritin or released using ferroportin (FPN). (b) During infection, bacterial recognition by pattern recognition receptors triggers activation of Toll-like receptors (TLR) and secretion of pro-inflammatory cytokines. As a result, cell release hepcidin to control iron release by FPN. Proinflammatory cyctokines are also involved in the regulation of Nramp1, which together with FPN deprives internalized bacteria of iron. TNF-α, IL-1 and IL-6 also stimulate the storage of iron using ferritin. (c) During infection, bacteria can lyse erythrocytes using various hemolysins and toxins to release heme and hemoglobin. The host re-captures these iron-rich proteins using haptoglobin and hemopexin, while P. aeruginosa secretes hemophores such as HasA for heme scavenging. (d) The host uses lactoferrin and transferrin to limit iron availability during infection. P. aeruginosa secretes siderophores with a high iron affinity such as pyoverdine to circumvent iron sequestration. In response, the host releases siderocalin/lipocalin-2 to neutralize bacterial siderophores. Elements that were found to be up-regulated during P. aeruginosa infection in the lung are indicated in bold black letters. Elements that were unchanged are shown in grey.