SUMMARY
Purpose
The aim of the present study is to demonstrate the efficacy of HyBeNX® to decontaminate the implant surface, both in the case mucositis and severe peri-implantitis and to allow future bone regeneration.
Materials and methods
We describe three case reports of peri-implantitis successfully treated with HyBeNX®. In our study, we have used microbiological tests to demonstrate the efficacy of HyBeNX® in decreasing bacterial load.
Results
The microbiological results of the clinical cases described show that there was a reduction in the total bacterial count after treatment.
Conclusions
The ability of HyBeNX® to dry the surface and remove biofilm may explain the efficacy of the decontamination and subsequent clinical improvements in all three cases.
Keywords: implant dentistry, peri-implantitis, mucositis, decontamination, hybenx
Introduction
During the last few years, dentistry has undergone a constant evolution, mainly due to the development of implantology. Thanks to this knowledge, it is possible to have numerous ready solutions to resolve cases of edentulism in patients.
Moreover, the continual increase in life expectancy has made it necessary to provide treatment for patients with severely atrophic crestal ridges and in patients with less than optimal physical health. The patient’s ability to manage implant hygiene can change over time in relation to their age and their state of physical health.
In the future, as the amount of implant procedures increase, there will be a direct correlation with the increase in the number of cases of peri-implantitis that dental professionals will need to treat.
Current available literature indicates that peri-implant infection, related to the biofilm colonization of the implant surface, which induces an inflammatory response, is one of the major causes of implant failure (1). There are two types of infection: the first affects only the soft tissues (peri-implant mucositis) and the second, which results in the loss of supporting bone (peri-implantitis) (2). It is estimated that approximately 10% of all implants positioned will develop peri-implant mucositis or peri-implantitis. Peri-implant mucositis can normally be resolved by debriding the area along with increased attention to personal oral hygiene by the patient.
The presence of bacteria in the soft-tissues can cause chronic inflammation that leads to bone resorption, thereby creating a pocket of space around the implant surface and the bone. This pocket can then be colonized by bacteria, and the biofilm left on the implant surface prevents bone reattachment. Up to now, there are no human studies demonstrating, on a histological level, the reattachment of bone to implant surface after peri-implantitis.
The lack of a specific clinical and radiographic definition of peri-implantitis makes it difficult to determine exactly how prevalent the disease is. The estimated number of cases has ranged from 5% to greater than 60%. The important point to note is that this disease is ubiquitous and the number of implants affected increases the longer these implants have been in place (3). In a recently published systematic review, in which 1497 patients and 6283 implants were followed for more than five years, peri-implant mucositis was found in 63.4% of patients and approximately 30.7% of the implants, and peri-implantitis was found in 18.8% of patients and 9.6% of implants, with smokers being at a higher risk of these conditions (4).
Current evidence suggests that peri-implantitis does not respond to traditional non-surgical therapy (5). In addition, surgical treatment has been demonstrated to result in significantly reduced probing depth and increasing gains in clinical attachment levels around affected implants (6).
Many approaches have been suggested to detoxify these surfaces (7), including mechanical methods, chemicals, laser and photodynamic therapies.
Among the mechanical methods, there is implantoplasty, the use of air powder abrasion, an ultrasonic scaler with a metal tip, and metallic and non-metallic curettes.
Implantoplasty, using rotary instruments may be indicated to completely flatten or smooth the exposed part of the titanium implant, which is open to the oral cavity and contaminated with bacteria (8). It has been demonstrated that rough surfaces accumulate more plaque than smooth or moderately rough surfaces (9–11). Therefore, maintaining a smooth, polished exterior surface of the implant may help reduce the reoccurrence of peri-implantitis.
Air powder abrasion (AP) features the use of an abrasive powder, generally sodium bicarbonate, and sodium hydrocarbonate (12), or amino acid glycine (13), propelled by a stream of compressed air to remove biofilm or extrinsic stains from the teeth (14).
This instrument applies a mix of water, air, and powder at pressures ranging from 65 to 100 pounds per square inch (psi) (15) and the efficacy of this approach has been demonstrated in both in vitro and in vivo studies in cleaning the implant surface as well as the clinical response to implants treated using this method.
Among the various chemical methods, citric acid (CA) has been widely reported in literature to detoxify the implant surface. However, to date, there has been no agreement as to the most effective concentration of the acid or how long the application should last during a treatment.
Chlorhexidine gluconate is the most important antiseptic used in periodontics (16).
Multiple studies have been published regarding its use in decontaminating an implant surface affected by peri-implantitis.
EDTA is also used in periodontics to remove the smear layer before applying biomimetic materials to facilitate regeneration.
Hydrogen Peroxide can also be used for this purpose. A 3% solution of Hydrogen Peroxide applied for 1 minute was shown to significantly decrease the amount of E. coli LPS present on the surface of a grit-blasted titanium alloy implant. However, Hydrogen Peroxide was shown to be the least effective when compared to the use of citric acid, plastic scalers, sonic tips and air powder abrasives (17).
Human studies have shown that combining implant surface cleaning with mechanical cleaning methods using curettes and saline soaked cotton pellets contributes to obtaining clinically stable results in implants followed for 24 months (18–20).
Tetracycline is a bacteriostatic antibiotic that inhibits protein synthesis. Case reports in humans have shown that 50 mg/mL of Tetracycline applied for 5 minutes after implantoplasty or AP and followed by an autogenous bone graft or xenograft and membrane resulted in the arrest of the disease and radiographic bone fill of the peri-implant defects (21–23).
In humans, the use of Erbium-doped Yttrium Aluminium Garnet (Er: YAG) laser showed no significance differences to the improvement of clinical parameters, such as bleeding on probing (BOP), pocket depth (PD) reduction of clinical attachment level (CAL) or bone fill, when compared with saline soaked cotton pellets at 12 and 24 months follow-up (19, 20).
Under dry conditions, a continuous CO2 laser has been shown to burn the contaminants but not to remove them (24). Continuous CO2 lasers used under wet conditions appear to be more successful, but still fail to remove all the contaminants and to restore the implant surface composition (24).
Little or no data are available on the other types of lasers for treating peri-implantitis.
Dentistry Photodynamic Therapy (PDT) is based on the application of photosensitive dyes, activated by a light with a specific wavelength, to kill bacteria (25).
It includes three basic elements: visible harmless light, a nontoxic photosensitizer, and oxygen.
Among these clinical treatment options, HYBENX ® has recently been proposed as a decontaminant gel (26–28).
HYBENX® Oral Tissue Decontaminant (EPIEN Medical s.r.l.) is a concentrated mixture of acidified phenolics, a desiccating agent, consisting of 60% sulfonated phenolics, 28% sulfuric acid, and 12% water. This product is an adjunctive agent to assist in the removal of plaque biofilm associated with standard mechanical dental hygiene procedures. Porter et al. have demonstrated the effectiveness of HYBENX® in treating recurrent oral aphthous ulcers (26).
The aim of the present study is to demonstrate the efficacy of HYBENX® to decontaminate the implant surface, both in the case mucositis and severe peri-implantitis and to allow future bone regeneration. In our study, we have used microbiological tests to demonstrate the efficacy of HYBENX® in decreasing bacterial load.
Case reports
In order to better illustrate the procedure used and the efficacy of the treatment, three clinical cases will be examined where each of the patients received treatment for peri-implantitis using HYBENX®, while the approach to the treatment protocol varies according to the clinical situation or pathology of the patient.
Microbiological sampling was carried out to test the total bacterial load and the quantity of the red complex present in the deepest pocket. In order to demonstrate both the immediate and long-term benefits of treatment with HYBENX®, microbiological testing was done on the same site before the detoxifying procedure, immediately after, and during the post-operative check-up, three months later.
Clinical case 1
A 68-year-old male patient presented with a severe case of peri-implantitis on the fixture in position 23. The patient, a non-smoker, was cardiopathic and being treated with hypertensive. He had previously been fitted with seven implants in the maxilla.
The patient received treatment for the decontamination of the implant, which had a sandblasted and acid-etched (SLA) surface.
A relief incision was made in zone 22 and another in zone 24 in order to open a flap. The implant was surrounded by chronically inflamed tissue and showed serious three-dimensional bone loss around the fixture (Figure 1). The first step in the procedure was to debride the newly exposed implant using diamond burs to smooth and polish the surface. Following this, a Teflon curette was used to further polish and finish the surface. Then a wash of 10 vol. Hydrogen Peroxide was applied to the implant surface, followed immediately by a rinse with physiological solution to remove all traces of the Hydrogen Peroxide solution.
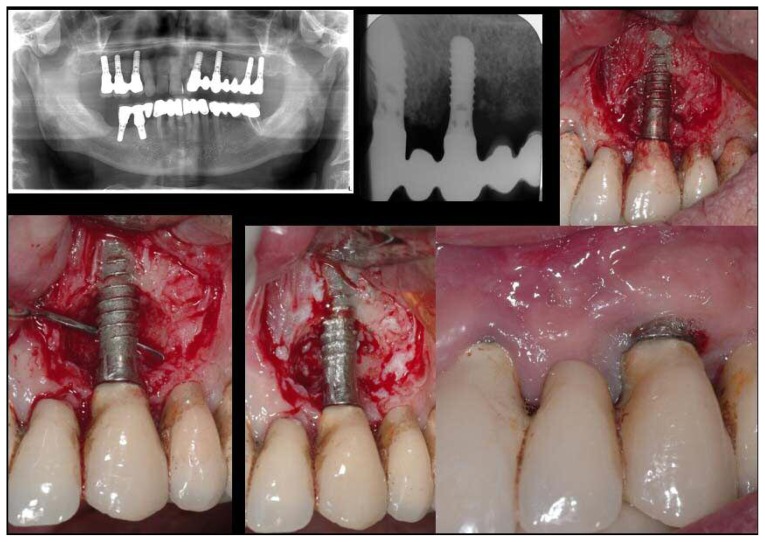
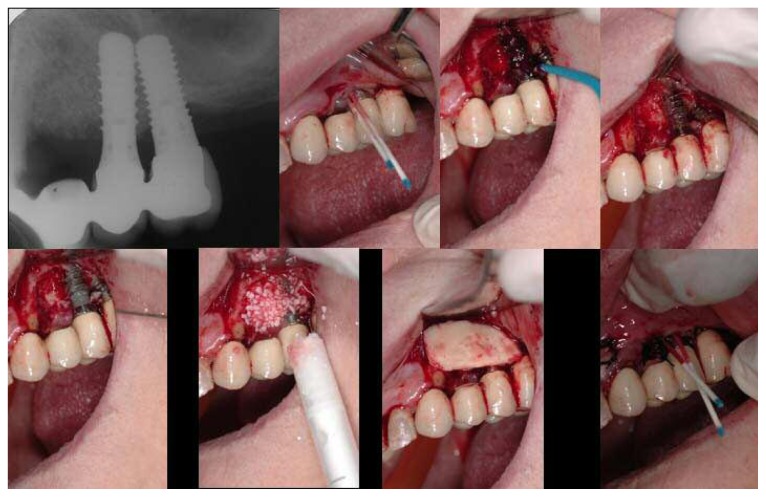
Figure 1.
Left - Right. Top: pre-operative OPT; endoral X-ray; clinical image showing inflamed tissues around the implant. Bottom: clinical image after debriding, showing significant bone loss before treatment; clinical image immediately after treatment; clinical image taken during 14-day post-operative check-up.
This first decontamination treatment, while providing immediate results, did not have a long-term positive outcome. Six months later, the site presented a second case of severe peri-implantitis with a mesial-distal pocket of 9 mm. There was the presence of a vestibular fistula with suppuration. It was clear that a different approach was necessary to solve the problem.
Microbiological sampling LABtest® (LAB SRL®, Ferrara, Italy), was carried out before the second treatment as previously described (30, 31). The first stage of the treatment involved the injecting of HYBENX® gel onto the implant surface, below the mucous tissue, taking care to distribute it uniformly. After 10 seconds, the area was rinsed thoroughly with physiological solution in order to deactivate and completely remove the gel. Subsequently, a second microbiological sampling was performed in the same site (Figure 2). The patient was brought in for a check-up 15 days later, and three months after treatment further microbiological sampling was done, and a control radiograph was taken (Figure 3).
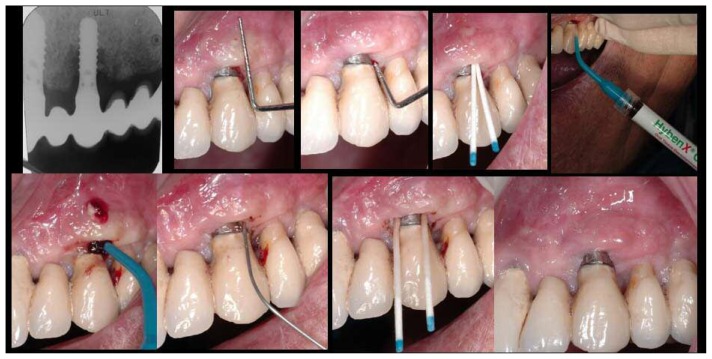
Figure 2.
HyBeNX® treatment images for clinical case 1. Left - Right. Top: pre-operative endoral X-ray; measurement of pocket depth (two images); microbiological test #1; HyBeNX® treatment. Bottom: Hy- BeNX® treatment showing fistula; physiological solution rinse; microbiological test #2; clinical image taken during three-month post-operative check-up.
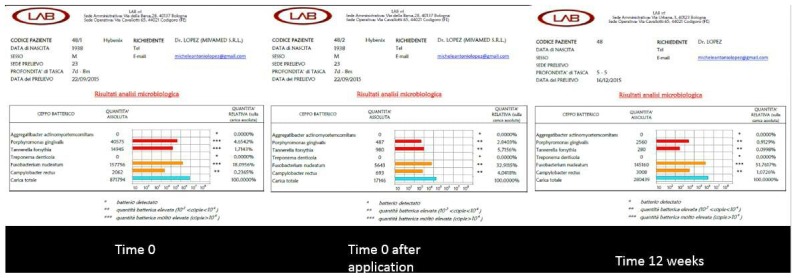
Figure 3.
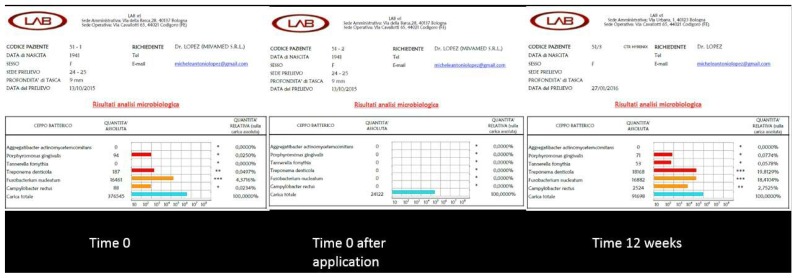
Results of the three microbiological tests for case 1.
Clinical case 2
The patient in case number 2 was a 59-year-old smoker, who was cardiopathic and being treated with antihypertensive and antiplatelet agents. He had two implants, in sites 25 and 26, which showed bone resorption between the fixtures, and a pocket of 6 mm, in the process of suppuration, was observed. Microbiological sampling of the site was carried out, which was subsequently injected with HYBENX® gel into the periodontal pocket and left to act for 10 seconds. The gel was then deactivated with several rinses of physiological solution, for 10 seconds, with a total of 10 ml of the solution being used. A second microbiological sampling was immediately taken. After three months, a third sampling test was performed to evaluate the long-term efficacy of the decontamination of the implant (Figure 4).
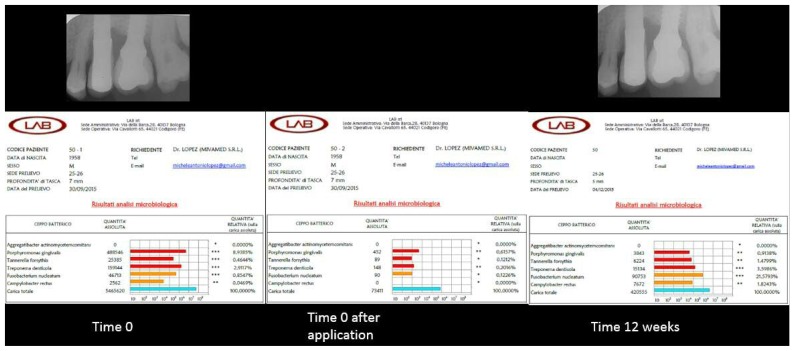
Figure 4.
Right - Left. Top: initial and final endoral X-rays for case number 2. Bottom: results of the three microbiological tests for case number 2.
Clinical case 3
A 73-year-old, female patient presented with a case of severe peri-implantitis on a fixture in position 25. Firstly, microbiological sampling of the site was carried out. Then, using an open flap approach, the implant surface was treated with HYBENX® gel using the same protocol as above (i.e. the gel was injected and left to act for 10 seconds and deactivated with rinses of physiological solution for 10 seconds). This was followed, immediately, with a guided bone regeneration (GBR) procedure, using pre-hydrated collagenated cortico-cancellous granules covered with a cortical lamina and pericardium membrane, both of porcine origin (ROEN Torino, Italy). A second microbiological sampling was immediately carried out. After three months, a third sampling was performed to evaluate the long-term efficacy of the decontamination of the implant (Figure 5).
Figure 5.
Right - Left. Top: pre-operative endoral X-ray; microbiological test #1; HyBeNX® treatment; physiological solution rinse. Bottom: implant surface after decontamination; GRB procedure with granules; GBR procedure with heterologous cortical lamina; microbiological test #2.
Results and discussion
The microbiological results of clinical case number 1 show that there had been a reduction of the total bacterial count immediately after treatment. It is evident how Porphyromonas gingivalis and Tannerella forsythia, elements of the red complex, were reduced quantitatively also in the check-up after three months, even if the total bacterial load tended to increase again. The peri-implant pocket was reduced to 5 mm and the vestibular fistula, which had been observed before treatment, was no longer present. Although the position of the implant presented a difficulty, due to the lack of sufficient bone thickness at the vestibular level, the treatment with HYBENX® allowed for a better control of peri-implantitis.
Also, in the case of patient number 2, treated using a flapless, non-invasive approach, the reduction of the red complex and of the total bacterial load was evident immediately after treatment, and was still found to be lower, according to the results of the microbiological test, at the 3-month post-operative check-up. The containment of the total bacterial load and the red complex explains the positive prognosis of this site. The peri-implant pocket was reduced from 6 to 4 mm.
In the third case, the possibility to treat the implant during open surgery allowed for an almost total elimination of bacteria and a regeneration (GBR) around the implant even in unfavorable conditions. Generally, a GBR procedure would not be recommended due to the bone loss present and the absence of bone peaks, as in the case of this patient, which are a requirement to the favourable outcome of such a procedure. The results of the second microbiological sampling, immediately after treatment, showed a total elimination of red complex bacteria.
The clinical situation observed during the postoperative check-up, after three months, showed a lack of suppuration. However, it should be noted that the results of the third and final sampling showed that both the bacterial load and the amount of red complex bacteria had increased. In cases such as this, the prognosis for the implant is bad, as the probability of peri-implantitis reoccurring is highly likely (Figure 6). Without further treatment, the implant would fail. In order to prevent implant failure or the removal of the implant, it is possible to use the non-invasive approach, as outline in case 2, using HYBENX ® as a decontamination treatment to keep peri-implantitis under control. There are several advantages to using this treatment method: minimum discomfort to the patient; the fact that, unlike in the case of administering medications such as Tetracycline, there is no risk of the bacteria developing antibiotic resistance; and the ease with which the procedure can be repeated, as necessary. The use of HYBENX® may influence prosthodontic (29–32) and endodontic (33, 34) clinical outcomes. In addiction the use of general and local anesthesia may have side effects (35–38) and severe complications (39).
Figure 6.
Results of the three microbiological tests for case number 3.
Conclusions
The ability of HYBENX® to dry the surface and remove biofilm may explain the efficacy of the decontamination and subsequent clinical improvements in all three cases, as outlined above. No invasive surgery was necessary to improve the clinical condition in the second patient. This means that there is minimum discomfort, and little or no healing time required. HYBENX® treatment can therefore be used for repeated treatments in cases of severe or recurring peri-implantitis with efficacy and minimum invasiveness.
In the three cases considered in this study, a significant improvement was obtained from a microbiological point of view, resulting in a reduction in the total bacterial load and in the red complex amount. Furthermore, from a clinical point of view, there was a clear improvement.
References
- 1.Mombelli A, van Oosten MA, Schurch E, Jr, Land NP. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol. 1987;2(4):145–51. doi: 10.1111/j.1399-302x.1987.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 2.Froum SJ, Rosen PS. A proposed classification for peri-implantitis. Int J Periodontics Restorative Dent. 2012;32(5):533–40. [PubMed] [Google Scholar]
- 3.Klinge B, van Steenberghe D. Methodology. European Journal of Oral Implantology; Working group on treatment options for the maintenance of marginal bone around endosseous oral implants; Stockholm, Sweden. 8 and 9 September 2011; 2011. pp. 9–12. [PubMed] [Google Scholar]
- 4.Atieh MA, Alsabeeha NH, Faggion CM, Jr, Duncan WJ. The frequency of peri-implant diseases: a systematic review and meta-analysis. J Periodontol. 2013;84(11):1586–98. doi: 10.1902/jop.2012.120592. [DOI] [PubMed] [Google Scholar]
- 5.Romanos GE, Weitz D. Therapy of peri-implant diseases. Where is the evidence? The Journal of Evidence-Based Dental Practice. 2012;12(S3):204–08. doi: 10.1016/S1532-3382(12)70038-6. [DOI] [PubMed] [Google Scholar]
- 6.Faggion CM, Jr, Chambrone L, Listl S, Tu YK. Network meta-analysis for evaluating interventions in implant dentistry: the case of peri-implantitis treatment. Clin Implant Dent Relat Res. 2013;15(4):576–88. doi: 10.1111/j.1708-8208.2011.00384.x. [DOI] [PubMed] [Google Scholar]
- 7.Valderrama P, Wilson TG., Jr Detoxification of implant surfaces affected by peri-implant disease: an overview of surgical methods. Int J Dent. 2013;2013(740680) doi: 10.1155/2013/740680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louropoulou A, Slot DE, Van der Weijden FA. Titanium surface alterations following the use of different mechanical instruments: a systematic review. Clin Oral Implants Res. 2012;23(6):643–58. doi: 10.1111/j.1600-0501.2011.02208.x. [DOI] [PubMed] [Google Scholar]
- 9.Quirynen M, van der Mei HC, Bollen CM, Schotte A, Marechal M, Doornbusch GI, Naert I, Busscher HJ, van Steenberghe D. An in vivo study of the influence of the surface roughness of implants on the microbiology of supra- and subgingival plaque. J Dent Res. 1993;72(9):1304–9. doi: 10.1177/00220345930720090801. [DOI] [PubMed] [Google Scholar]
- 10.Subramani K, Jung RE, Molenberg A, Hammerle CH. Biofilm on dental implants: a review of the literature. Int J Oral Maxillofac Implants. 2009;24(4):616–26. [PubMed] [Google Scholar]
- 11.Romeo E, Ghisolfi M, Carmagnola D. Peri-implant diseases. A systematic review of the literature. Minerva Stomatol. 2004;53(5):215–30. [PubMed] [Google Scholar]
- 12.Augthun M, Tinschert J, Huber A. In vitro studies on the effect of cleaning methods on different implant surfaces. J Periodontol. 1998;69(8):857–64. doi: 10.1902/jop.1998.69.8.857. [DOI] [PubMed] [Google Scholar]
- 13.Schwarz F, Ferrari D, Popovski K, Hartig B, Becker J. Influence of different air-abrasive powders on cell viability at biologically contaminated titanium dental implants surfaces. J Biomed Mater Res B Appl Biomater. 2009;88(1):83–91. doi: 10.1002/jbm.b.31154. [DOI] [PubMed] [Google Scholar]
- 14.Tastepe CS, van Waas R, Liu Y, Wismeijer D. Air powder abrasive treatment as an implant surface cleaning method: a literature review. Int J Oral Maxillofac Implants. 2012;27(6):1461–73. [PubMed] [Google Scholar]
- 15.Finlayson RS, Stevens FD. Subcutaneous facial emphysema secondary to use of the Cavi-Jet. J Periodontol. 1988;59(5):315–7. doi: 10.1902/jop.1988.59.5.315. [DOI] [PubMed] [Google Scholar]
- 16.Cohen DW, Atlas SL. Chlorhexidine gluconate in periodontal treatment. Compend Suppl. 1994;18:S711–3. quiz S14–7. [PubMed] [Google Scholar]
- 17.Zablotsky MH, Diedrich DL, Meffert RM. Detoxification of endotoxin-contaminated titanium and hydroxyapatite-coated surfaces utilizing various chemo - therapeutic and mechanical modalities. Implant Dent. 1992;1(2):154–8. doi: 10.1097/00008505-199205000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Schwarz F, Sahm N, Becker J. Combined surgical therapy of advanced peri-implantitis lesions with concomitant soft tissue volume augmentation. A case series. Clin Oral Implants Res. 2014;25(1):132–6. doi: 10.1111/clr.12103. [DOI] [PubMed] [Google Scholar]
- 19.Schwarz F, Sahm N, Iglhaut G, Becker J. Impact of the method of surface debridement and decontamination on the clinical outcome following combined surgical therapy of peri-implantitis: a randomized controlled clinical study. J Clin Periodontol. 2011;38(3):276–84. doi: 10.1111/j.1600-051X.2010.01690.x. [DOI] [PubMed] [Google Scholar]
- 20.Schwarz F, John G, Mainusch S, Sahm N, Becker J. Combined surgical therapy of peri-implantitis evaluating two methods of surface debridement and decontamination. A two-year clinical follow up report. J Clin Periodontol. 2012;39(8):789–97. doi: 10.1111/j.1600-051X.2012.01867.x. [DOI] [PubMed] [Google Scholar]
- 21.Suh JJ, Simon Z, Jeon YS, Choi BG, Kim CK. The use of implantoplasty and guided bone regeneration in the treatment of peri-implantitis: two case reports. Implant Dent. 2003;12(4):277–82. doi: 10.1097/01.id.0000091139.04246.bc. [DOI] [PubMed] [Google Scholar]
- 22.Tinti C, Parma-Benfenati S. Treatment of peri-implant defects with the vertical ridge augmentation procedure: a patient report. Int J Oral Maxillofac Implants. 2001;16(4):572–7. [PubMed] [Google Scholar]
- 23.Park JB. Treatment of peri-implantitis with deproteinised bovine bone and tetracycline: a case report. Gerodontology. 2012;29(2):145–9. doi: 10.1111/j.1741-2358.2011.00470.x. [DOI] [PubMed] [Google Scholar]
- 24.Mouhyi J, Sennerby L, Pireaux JJ, Dourov N, Nammour S, Van Reck J. An XPS and SEM evaluation of six chemical and physical techniques for cleaning of contaminated titanium implants. Clin Oral Implants Res. 1998;9(3):185–94. doi: 10.1034/j.1600-0501.1998.090306.x. [DOI] [PubMed] [Google Scholar]
- 25.Schar D, Ramseier CA, Eick S, Arweiler NB, Sculean A, Salvi GE. Anti-infective therapy of peri-implantitis with adjunctive local drug delivery or photodynamic therapy: six-month outcomes of a prospective randomized clinical trial. Clin Oral Implants Res. 2013;24(1):104–10. doi: 10.1111/j.1600-0501.2012.02494.x. [DOI] [PubMed] [Google Scholar]
- 26.Porter SR, Al-Johani K, Fedele S, Moles DR. Randomised controlled trial of the efficacy of HybenX in the symptomatic treatment of recurrent aphthous stomatitis. Oral Dis. 2009;15(2):155–61. doi: 10.1111/j.1601-0825.2008.01503.x. [DOI] [PubMed] [Google Scholar]
- 27.Lombardo G, Signoretto C, Corrocher G, Pardo A, Pighi J, Rovera A, Caccuri F, Nocini PF. A topical desiccant agent in association with ultrasonic debridement in the initial treatment of chronic periodontitis: a clinical and microbiological study. New Microbiol. 2015;38(3):393–407. [PubMed] [Google Scholar]
- 28.Pini-Prato G, Magnani C, Rotundo R. Treatment of Acute Periodontal Abscesses Using the Biofilm Decontamination Approach: A Case Report Study. Int J Periodontics Restorative Dent. 2016 Jan-Feb;36(1):55–63. doi: 10.11607/prd.2557. [DOI] [PubMed] [Google Scholar]
- 29.Ottria L, Zavattini A, Ceruso FM, Gargari M. Maxillofacial prosthesis (P.M.F): in a case of oral-nasal communication post-surgery and post-radiotherapy. Oral Implantol (Rome) 2014;7(2):46–50. [PMC free article] [PubMed] [Google Scholar]
- 30.Gargari M, Gloria F, Cappello A, Ottria L. Strength of zirconia fixed partial dentures: review of the literature. Oral Implantol (Rome) 2010;3(4):15–24. [PMC free article] [PubMed] [Google Scholar]
- 31.De Vico G, Ottria L, Bollero P, Bonino M, Cialone M, Barlattani A, Jr, Gargari M. Aesthetic and functionality in fixed prosthodontic: sperimental and clinical analysis of the CAD-CAM systematic 3Shape. Oral Implantol (Rome) 2008;1(3–4):104–15. [PMC free article] [PubMed] [Google Scholar]
- 32.Moretto D, Gargari M, Nordsjo E, Gloria F, Ottria L. Immediate loading: a new implant technique with immediate loading and aesthetics: Nobel Active. Oral Implantol (Rome) 2008;1(2):50–5. [PMC free article] [PubMed] [Google Scholar]
- 33.Fanucci E, Nezzo M, Neroni L, Montesani L, Jr, Ottria L, Gargari M. Diagnosis and treatment of paranasal sinus fungus ball of odontogenic origin: case report. Oral Implantol (Rome) 2013;6(3):63–6. [PMC free article] [PubMed] [Google Scholar]
- 34.Gargari M, Ottria L, Nezzo M, Neroni L, Fanucci E. Cone Beam CT use in the pre-prosthetic evaluation of endodontically treated of the rear maxilla. Oral Implantol (Rome) 2012;5(2–3):42–6. [PMC free article] [PubMed] [Google Scholar]
- 35.Feltracco P, Gaudio RM, Barbieri S, Tiano L, Iacobone M, Viel G, Tonetti T, Galligioni H, Bortolato A, Ori C, Avato FM. The perils of dental vacation: possible anaesthetic and medicolegal consequences. Med Sci Law. 2013;53(1):19–23. doi: 10.1258/msl.2012.012047. [DOI] [PubMed] [Google Scholar]
- 36.Feltracco P, Barbieri S, Galligioni H, Pasin L, Gaudio RM, Tommasi A, Zucchetto A, Trevisiol P, Ori C, Avato FM. A fatal case of anaphylactic shock during paragliding. J Forensic Sci. 2012;57(6):1656–8. doi: 10.1111/j.1556-4029.2012.02187.x. [DOI] [PubMed] [Google Scholar]
- 37.Feltracco P, Gaudio RM, Avato FM, Ori C. Authors’ Response (Letter) Journal of Forensic Sciences. 2012;57(5) doi: 10.1111/j.1556-4029.2012.02187.x. [DOI] [PubMed] [Google Scholar]
- 38.Gaudio RM, Barbieri S, Feltracco P, Tiano L, Galligioni H, Uberti M, Ori C, Avato FM. Traumatic dental injuries during anaesthesia. Part II: medico-legal evaluation and liability. Dent Traumatol. 2011;27(1):40–5. doi: 10.1111/j.1600-9657.2010.00956.x. [DOI] [PubMed] [Google Scholar]
- 39.Gaudio RM, Barbieri S, Feltracco P, Spaziani F, Alberti M, Delantone M, Trevisiol P, Righini F, Talarico A, Sanchioni R, Spagna A, Pietrantonio V, Zilio G, Dalla Valle R, Vettore G, Montisci M, Bortoluzzi A, Sacco A, Ramacciato G, Pasetti A, Mognato E, Ferronato C, Costola A, Ori C, Avato FM. Impact of alcohol consumption on winter sports-related injuries. Med Sci Law. 2010;50(3):122–5. doi: 10.1258/msl.2010.010007. [DOI] [PubMed] [Google Scholar]