Abstract
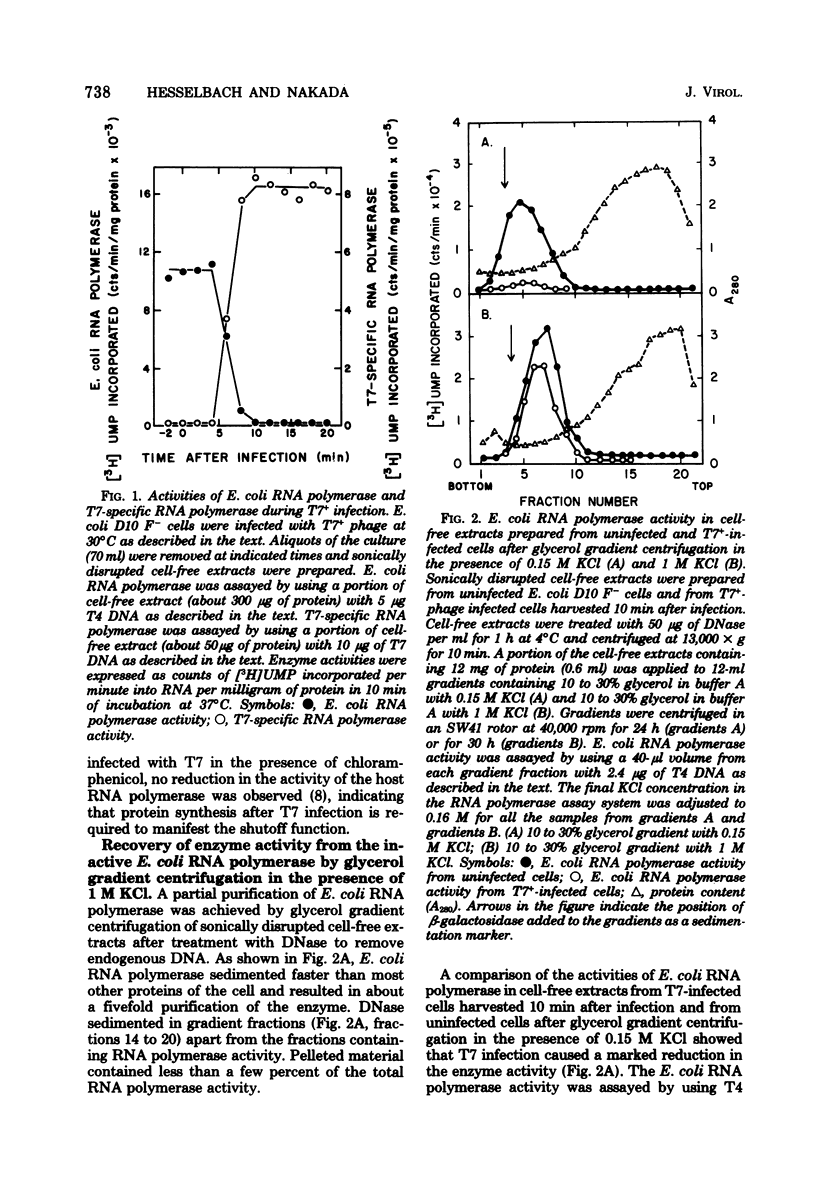
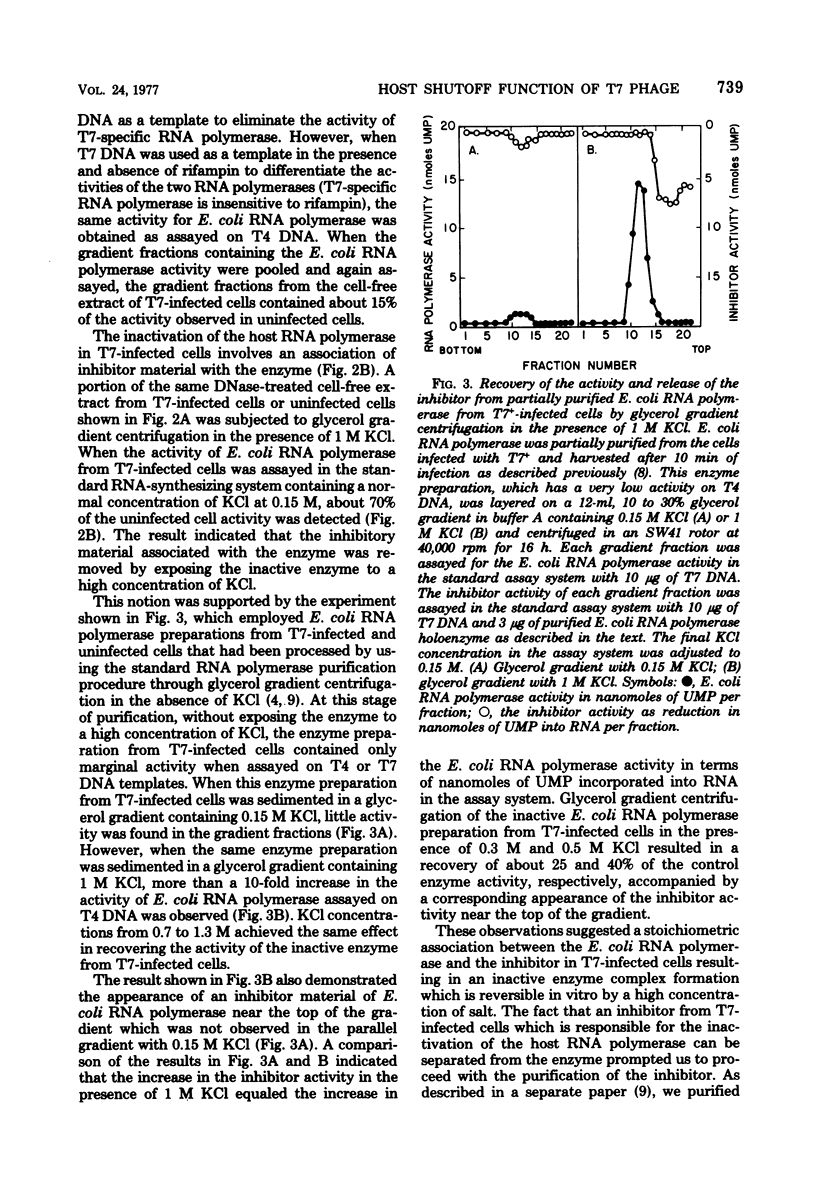
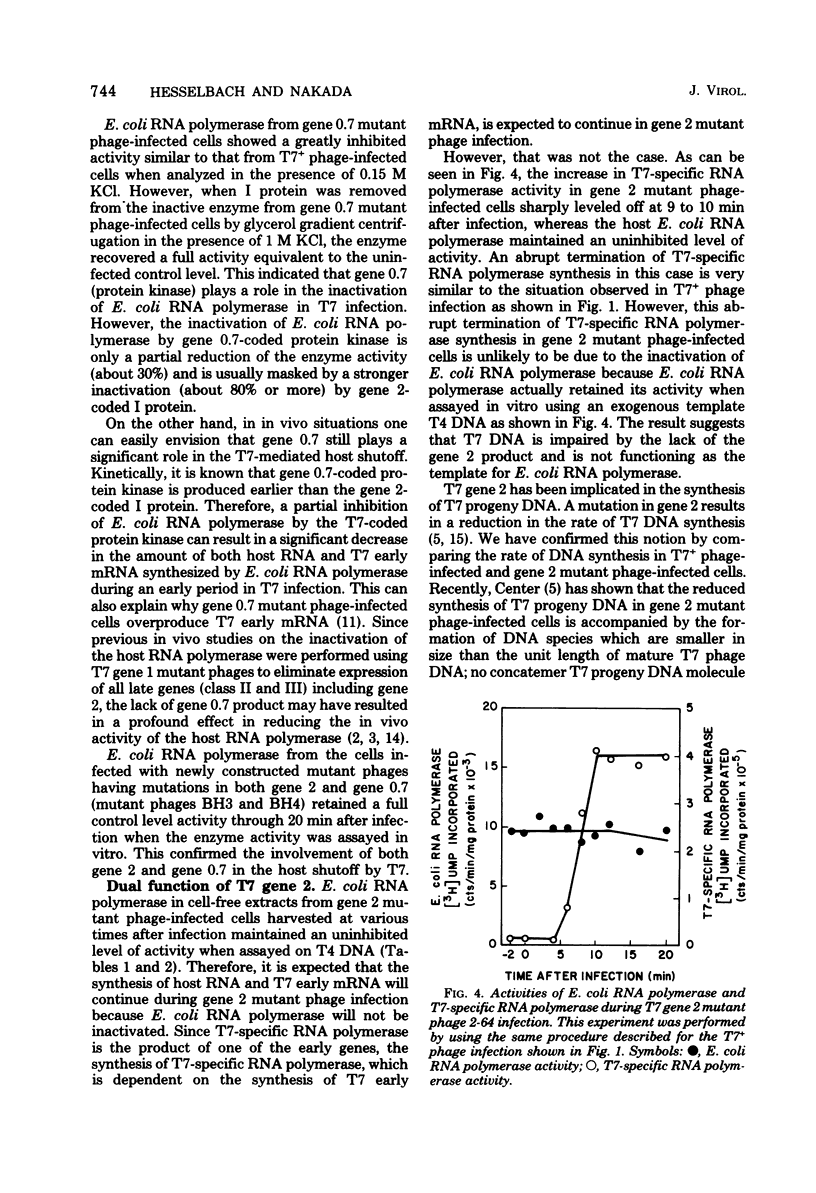
The “host shutoff” function of bacteriophage T7 involves an inactivation of the host Escherichia coli RNA polymerase by an inhibitor protein bound to the enzyme. When this inhibitor protein, termed I protein, was removed from the inactive RNA polymerase complex prepared from T7-infected cells by glycerol gradient centrifugation in the presence of 1 M KCl, the enzyme recovered its activity equivalent to about 70 to 80% of the activity of the enzyme from uninfected cells. Analysis of the activity of E. coli RNA polymerase from E. coli cells infected with various T7 mutant phages indicated that the T7 gene 2 codes for the inhibitor I protein. The activity of E. coli RNA polymerase from gene 2 mutant phage-infected cells, which was about 70% of that from uninfected cells, did not increase after glycerol gradient centrifugation in the presence of 1 M KCl, indicating that the salt-removable inhibitor was not present with the enzyme. It was found that the reduction in E. coli RNA polymerase activity in cells infected with T7+ or gene 2 mutant phage, i.e., about 70% of the activity of the enzyme compared to that from uninfected cells after glycerol gradient centrifugation in the presence of 1 M KCl, results from the function of T7 gene 0.7. E. coli RNA polymerase from gene 0.7 mutant phage-infected cells was inactive but recovered a full activity equivalent to that from uninfected cells after removal of the inhibitor I protein with 1 M KCl. E. coli RNA polymerase from the cells infected with newly constructed mutant phages having mutations in both gene 2 and gene 0.7 retained the full activity equivalent to that from uninfected cells with or without treatment of the enzyme with 1 M KCl. From these results, we conclude that both gene 2 and gene 0.7 of T7 are involved in accomplishing complete shutoff of the host E. coli RNA polymerase activity in T7 infection.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunovskis I., Summers W. C. The process of infection with coliphage 17. VI. A phage gene controlling shutoff of host RNA synthesis. Virology. 1972 Nov;50(2):322–327. doi: 10.1016/0042-6822(72)90383-2. [DOI] [PubMed] [Google Scholar]
- Brunovskis I., Summers W. C. The process of infection with coliphage T7. V. Shutoff of host RNA synthesis by an early phage function. Virology. 1971 Jul;45(1):224–231. doi: 10.1016/0042-6822(71)90129-2. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- Center M. S. Role of gene 2 in bacteriophage T7 DNA synthesis. J Virol. 1975 Jul;16(1):94–100. doi: 10.1128/jvi.16.1.94-100.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin M., McGrath J., Waskell L. New RNA polymerase from Escherichia coli infected with bacteriophage T7. Nature. 1970 Oct 17;228(5268):227–231. doi: 10.1038/228227a0. [DOI] [PubMed] [Google Scholar]
- Hausmann R. Bacteriophage T7 genetics. Curr Top Microbiol Immunol. 1976;75:77–110. doi: 10.1007/978-3-642-66530-1_3. [DOI] [PubMed] [Google Scholar]
- Hesselbach B. A., Nakada D. I protein: bacteriophage T7-coded inhibitor of Escherichia coli RNA polymerase. J Virol. 1977 Dec;24(3):746–760. doi: 10.1128/jvi.24.3.746-760.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselbach B. A., Nakada D. Inactive complex formation between E. coli RNA polymerase and inhibitor protein purified from T7 phage infected cells. Nature. 1975 Nov 27;258(5533):354–357. doi: 10.1038/258354a0. [DOI] [PubMed] [Google Scholar]
- Hesselbach B. A., Yamada Y., Nakada D. Isolation of an inhibitor protein of E. coli RNA polymerase from T7 phage infected cell. Nature. 1974 Nov 1;252(5478):71–74. doi: 10.1038/252071b0. [DOI] [PubMed] [Google Scholar]
- Ponta H., Rahmsdorf H. J., Pai S. H., Hirsch-Kauffmann M., Herrlich P., Schweiger M. Control of gene expression in bacteriophage T7: transcriptional controls. Mol Gen Genet. 1974;134(4):281–297. doi: 10.1007/BF00337463. [DOI] [PubMed] [Google Scholar]
- Rahmsdorf H. J., Pai S. H., Ponta H., Herrlich P., Roskoski R., Jr, Schweiger M., Studier F. W. Protein kinase induction in Escherichia coli by bacteriophage T7. Proc Natl Acad Sci U S A. 1974 Feb;71(2):586–589. doi: 10.1073/pnas.71.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner D. The interaction bacterial and phage proteins with immobilized Escherichia coli RNA polymerase. J Mol Biol. 1974 Sep 15;88(2):373–383. doi: 10.1016/0022-2836(74)90488-4. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Gene 0.3 of bacteriophage T7 acts to overcome the DNA restriction system of the host. J Mol Biol. 1975 May 15;94(2):283–295. doi: 10.1016/0022-2836(75)90083-2. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Genetic analysis of non-essential bacteriophage T7 genes. J Mol Biol. 1973 Sep 15;79(2):227–236. doi: 10.1016/0022-2836(73)90002-8. [DOI] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]
- Whitaker P. A., Yamada Y., Nakada D. F-Factor-mediated restriction of bacteriophage T7: synthesis of RNA and protein in T7-infected Escherichia coli F- and F+ cells. J Virol. 1975 Dec;16(6):1380–1390. doi: 10.1128/jvi.16.6.1380-1390.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Whitaker P. A., Nakada D. Early to late switch in bacteriophage T7 development: functional decay of T7 early messenger RNA. J Mol Biol. 1974 Oct 25;89(2):293–303. doi: 10.1016/0022-2836(74)90520-8. [DOI] [PubMed] [Google Scholar]
- Zillig W., Fujiki H., Blum W., Janeković D., Schweiger M., Rahmsdorf H., Ponta H., Hirsch-Kauffmann M. In vivo and in vitro phosphorylation of DNA-dependent RNA polymerase of Escherichia coli by bacteriophage-T7-induced protein kinase. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2506–2510. doi: 10.1073/pnas.72.7.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]


