Fig. 4.
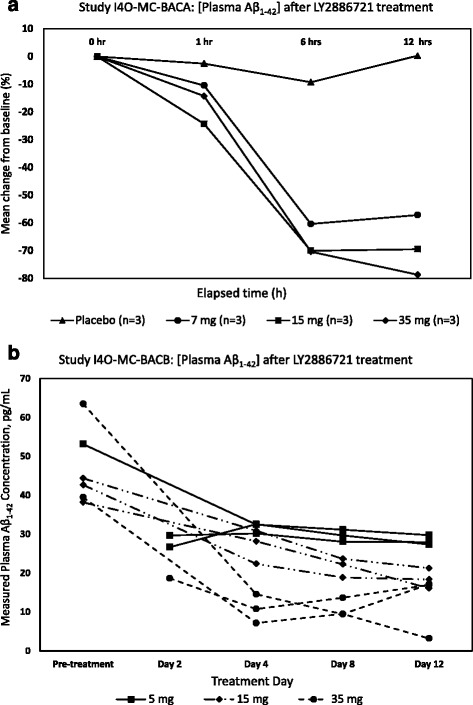
Changes in plasma amyloid-β 1–42 peptide (Aβ1–42) concentration in subjects who received oral doses of the β-site amyloid precursor protein cleaving enzyme 1inhibitor LY2886721 in separate clinical studies. a Mean percentage change in Aβ1–42 concentration from baseline values for subjects treated with single doses of LY2886721 in study I4O-MC-BACA. b Plasma Aβ1–42 concentration for individual subjects treated with 14 consecutive once-daily doses of LY2886721 in study I4O-MC-BACB. Samples from both studies were quantified with Fujirebio Aβ1–42 peptide standard
