Abstract
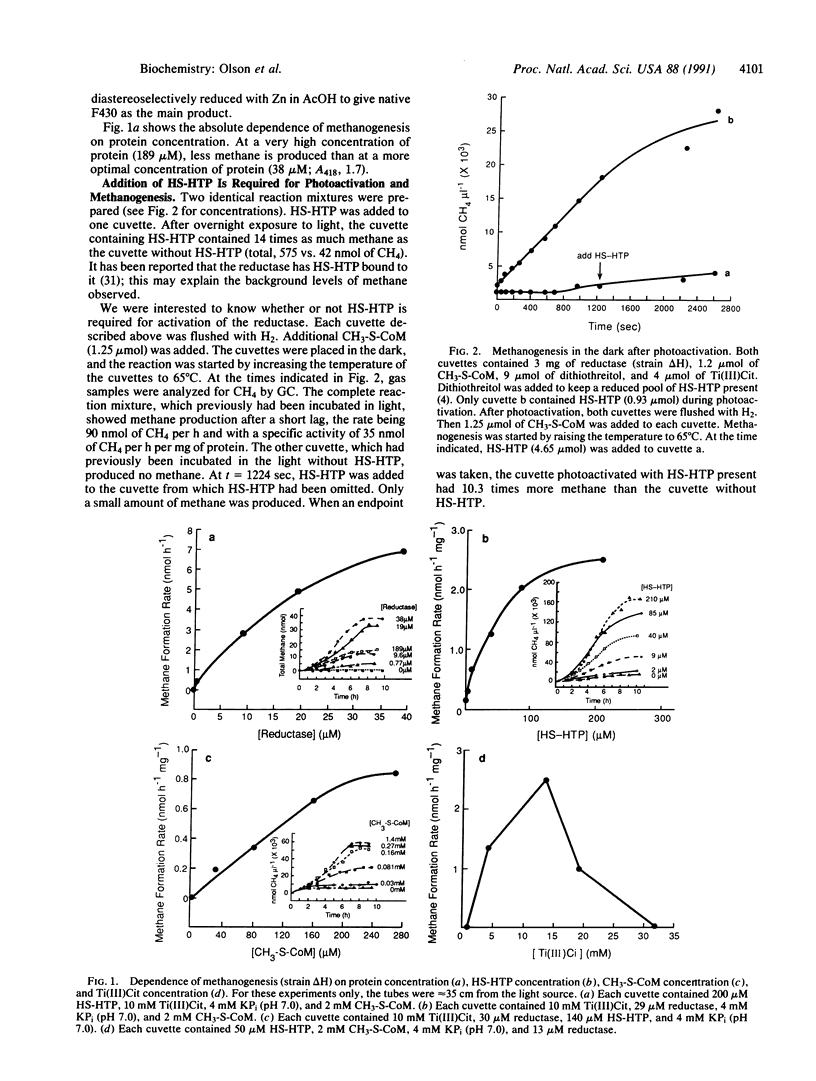
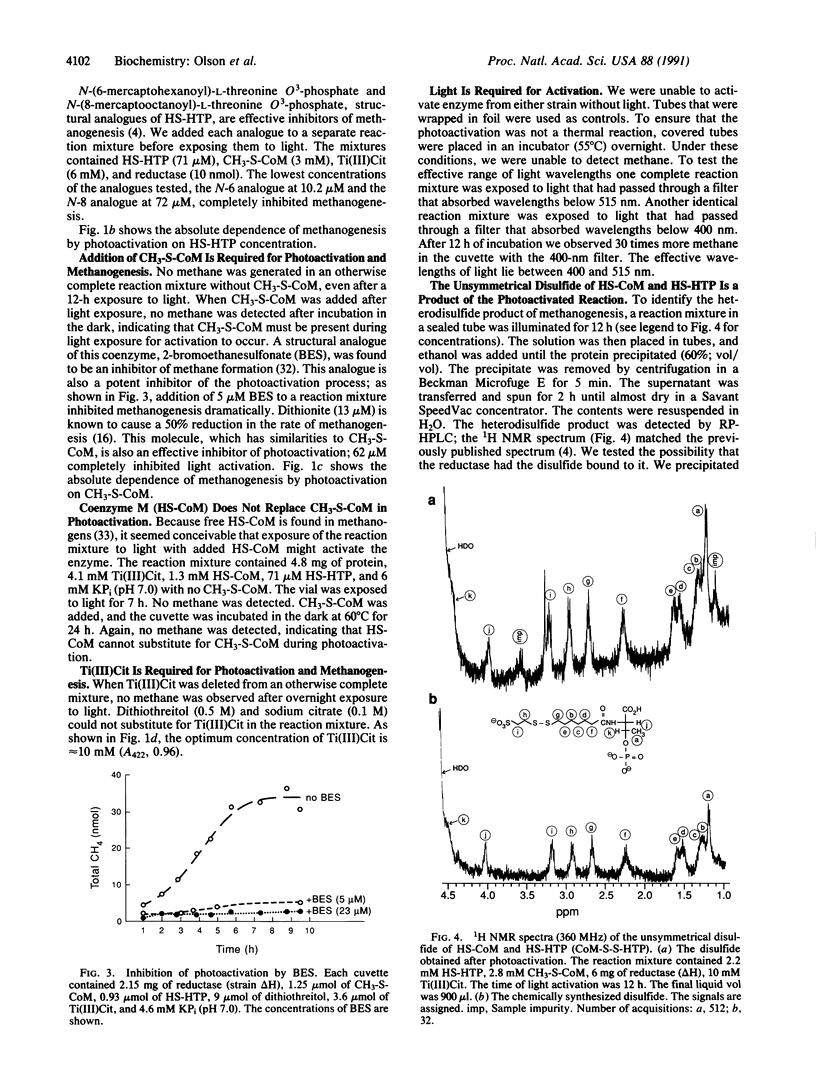
Inactive 2-(methylthio)ethanesulfonic acid (CH3-S-CoM) reductase was partially activated by exposure to light. This simplified system replaces the complex enzymatic system of protein components A2, A3a, A3b, and ATP, which previously represented the only available means of reactivating the enzyme. Components necessary for light activation include N-(7-mercaptoheptanoyl)-L-threonine O3-phosphate (HS-HTP), CH3-S-CoM, titanium(III) citrate [Ti(III)Cit], and light above 400 nm. Photoactivation was inhibited by known inhibitors of methanogenesis: 2-bromoethanesulfonate (BES), N-(6-mercaptohexanoyl)-L-threonine O3-phosphate, N-(8-mercaptooctanoyl)-L-threonine O3-phosphate, and sodium dithionite. Methanogenesis continued when the light-activated reaction mixture was incubated in the dark. Although the specific activity was low (35 nmol of CH4 per h per mg of protein) the reaction products methane and the unsymmetrical disulfide of 2-mercaptoethanesulfonate (HS-CoM) and HS-HTP were identified. We were unable to photoactivate a reaction mixture containing the isolated prosthetic group, native F430, or its analogues.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Ankel-Fuchs D., Böcher R., Thauer R. K., Noll K. M., Wolfe R. S. 7-Mercaptoheptanoylthreonine phosphate functions as component B in ATP-independent methane formation from methyl-CoM with reduced cobalamin as electron donor. FEBS Lett. 1987 Mar 9;213(1):123–127. doi: 10.1016/0014-5793(87)81476-x. [DOI] [PubMed] [Google Scholar]
- Balch W. E., Wolfe R. S. Specificity and biological distribution of coenzyme M (2-mercaptoethanesulfonic acid). J Bacteriol. 1979 Jan;137(1):256–263. doi: 10.1128/jb.137.1.256-263.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobik T. A., Olson K. D., Noll K. M., Wolfe R. S. Evidence that the heterodisulfide of coenzyme M and 7-mercaptoheptanoylthreonine phosphate is a product of the methylreductase reaction in Methanobacterium. Biochem Biophys Res Commun. 1987 Dec 16;149(2):455–460. doi: 10.1016/0006-291x(87)90389-5. [DOI] [PubMed] [Google Scholar]
- Bobik T. A., Wolfe R. S. Physiological importance of the heterodisulfide of coenzyme M and 7-mercaptoheptanoylthreonine phosphate in the reduction of carbon dioxide to methane in Methanobacterium. Proc Natl Acad Sci U S A. 1988 Jan;85(1):60–63. doi: 10.1073/pnas.85.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco A. A., Bobik T. A., Wolfe R. S. Unusual coenzymes of methanogenesis. Annu Rev Biochem. 1990;59:355–394. doi: 10.1146/annurev.bi.59.070190.002035. [DOI] [PubMed] [Google Scholar]
- Diekert G., Konheiser U., Piechulla K., Thauer R. K. Nickel requirement and factor F430 content of methanogenic bacteria. J Bacteriol. 1981 Nov;148(2):459–464. doi: 10.1128/jb.148.2.459-464.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. A colorimetric method for determining low concentrations of mercaptans. Arch Biochem Biophys. 1958 Apr;74(2):443–450. doi: 10.1016/0003-9861(58)90014-6. [DOI] [PubMed] [Google Scholar]
- Ellefson W. L., Wolfe R. S. Component C of the methylreductase system of Methanobacterium. J Biol Chem. 1981 May 10;256(9):4259–4262. [PubMed] [Google Scholar]
- Ellermann J., Hedderich R., Böcher R., Thauer R. K. The final step in methane formation. Investigations with highly purified methyl-CoM reductase (component C) from Methanobacterium thermoautotrophicum (strain Marburg). Eur J Biochem. 1988 Mar 15;172(3):669–677. doi: 10.1111/j.1432-1033.1988.tb13941.x. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gunsalus R. P., Romesser J. A., Wolfe R. S. Preparation of coenzyme M analogues and their activity in the methyl coenzyme M reductase system of Methanobacterium thermoautotrophicum. Biochemistry. 1978 Jun 13;17(12):2374–2377. doi: 10.1021/bi00605a019. [DOI] [PubMed] [Google Scholar]
- Hartzell P. L., Escalante-Semerena J. C., Bobik T. A., Wolfe R. S. A simplified methylcoenzyme M methylreductase assay with artificial electron donors and different preparations of component C from Methanobacterium thermoautotrophicum delta H. J Bacteriol. 1988 Jun;170(6):2711–2715. doi: 10.1128/jb.170.6.2711-2715.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. A., Pickard M. D. Effect of titanium (III) citrate as reducing agent on growth of rumen bacteria. Appl Environ Microbiol. 1980 Jun;39(6):1144–1147. doi: 10.1128/aem.39.6.1144-1147.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W. J., Nagle D. P., Jr, Whitman W. B. Methanogens and the diversity of archaebacteria. Microbiol Rev. 1987 Mar;51(1):135–177. doi: 10.1128/mr.51.1.135-177.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krone U. E., Laufer K., Thauer R. K., Hogenkamp H. P. Coenzyme F430 as a possible catalyst for the reductive dehalogenation of chlorinated C1 hydrocarbons in methanogenic bacteria. Biochemistry. 1989 Dec 26;28(26):10061–10065. doi: 10.1021/bi00452a027. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leigh J. A., Wolfe R. S. Carbon dioxide reduction factor and methanopterin, two coenzymes required for CO2 reduction to methane by extracts of Methanobacterium. J Biol Chem. 1983 Jun 25;258(12):7536–7540. [PubMed] [Google Scholar]
- Noll K. M., Donnelly M. I., Wolfe R. S. Synthesis of 7-mercaptoheptanoylthreonine phosphate and its activity in the methylcoenzyme M methylreductase system. J Biol Chem. 1987 Jan 15;262(2):513–515. [PubMed] [Google Scholar]
- Noll K. M., Wolfe R. S. Component C of the methylcoenzyme M methylreductase system contains bound 7-mercaptoheptanoylthreonine phosphate (HS-HTP). Biochem Biophys Res Commun. 1986 Sep 30;139(3):889–895. doi: 10.1016/s0006-291x(86)80261-3. [DOI] [PubMed] [Google Scholar]
- Romesser J. A., Balch W. E. Coenzyme M: preparation and assay. Methods Enzymol. 1980;67:545–552. doi: 10.1016/s0076-6879(80)67067-0. [DOI] [PubMed] [Google Scholar]
- Rouvière P. E., Bobik T. A., Wolfe R. S. Reductive activation of the methyl coenzyme M methylreductase system of Methanobacterium thermoautotrophicum delta H. J Bacteriol. 1988 Sep;170(9):3946–3952. doi: 10.1128/jb.170.9.3946-3952.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiemke A. K., Hamilton C. L., Scott R. A. Structural heterogeneity and purification of protein-free F430 from the cytoplasm of Methanobacterium thermoautotrophicum. J Biol Chem. 1988 Apr 25;263(12):5611–5616. [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]


