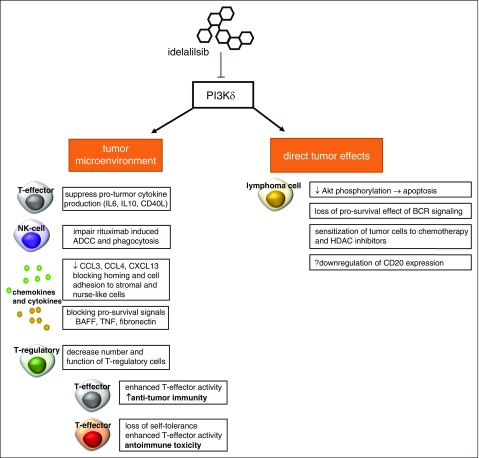
Figure 1.
Potential effects of idelalisib. Selective inhibition of PI3Kδ does appear to act directly on lymphoma cells, reducing Akt phosphorylation limitation activation of the mTOR/Akt and NF-κN pathways. This leads to apoptosis through a caspase-dependent mechanism. Prosurvival signals from the B-cell receptor via PI3K are abrogated by idelalisib. In addition, it appears that idelalisib sensitizes malignant B cells to both chemotherapy and histone deacetylase inhibitors. Idelalisib also exerts pleiotropic effects on the tumor microenvironment. T-cell cytokine production and release are governed in part by PI3K, and idelalisib appears to reduce pro-inflammatory cytokines such as IL-6, IL-10, and CD40L. Similar to ibrutinib, idelalisib alters chemokines and blocks adhesion of tumor cells to supporting stromal cells. To a lesser extent than ibrutinib, idelalisib has been shown to partially abrogate antibody-mediated cell mediate cytotoxicity induced by anti-CD20 monoclonal antibodies such as rituximab. Finally, recent data indicate idelalisib-treated patients who experience severe immune toxicity have decreased number and function T-regulatory cells in the peripheral blood. T-regulatory cells play a critical role in regulating the activity of T-effector cells; in the absence of function, deregulated T-effector cell activity can cause both increased antitumor immunity and loss of self-tolerance with unwanted autoimmune toxicity. ADCC, antibody-mediated cell mediate cytotoxicity; BAFF, B-cell activating factor; BCR, B-cell receptor; CCL3/4, chemokine ligand 3/4; CXCL13, chemokine ligand 13; HDAC, histone deacetylase; TNF, tumor necrosis factor.