Abstract
We examined the proportions of multiple types of breast cancers in the population that were attributable to established risk factors, focusing on behaviors that are modifiable at menopause. We estimated the full and partial population attributable risk percentages (PAR%) by combining the relative risks and the observed prevalence rates of the risk factors of interest. A total of 8,421 cases of invasive breast cancer developed in postmenopausal women (n = 121,700) in the Nurses’ Health Study from 1980–2010. We included the following modifiable risk factors in our analyses: weight change since age 18 years, alcohol consumption, physical activity level, breastfeeding, and menopausal hormone therapy use. Additionally, the following nonmodifiable factors were included: age, age at menarche, height, a combination of parity and age at first birth, body mass index at age 18 years, family history of breast cancer, and prior benign breast disease. When we considered all risk factors (and controlled for age), the PAR% for invasive breast cancers was 70.0% (95% confidence interval: 55.0, 80.7). When considering only modifiable factors, we found that changing the risk factor profile to the lowest weight gain, no alcohol consumption, high physical activity level, breastfeeding, and no menopausal hormone therapy use was associated with a PAR% of 34.6% (95% confidence interval: 22.7, 45.4). The PAR% for modifiable factors was higher for estrogen receptor–positive breast cancers (PAR% = 39.7%) than for estrogen receptor–negative breast cancers (PAR% = 27.9%). Risk factors that are modifiable at menopause account for more than one-third of postmenopausal breast cancers; therefore, a substantial proportion of breast cancer in the United States is preventable.
Keywords: modifiable factors, PAR%, postmenopausal breast cancer
Breast cancer is the most common cancer among women in the United States, and it is a major cause of morbidity and mortality globally. It has been estimated that 232,340 cases of invasive breast cancer and 39,620 breast cancer deaths occur annually in the United States (1). A nearly 5-fold difference in breast cancer incidence rates exists among countries globally (2). This geographic variability in incidence rates for breast cancer suggests that differences in the prevalence rates of risk factors among countries are associated with variation in breast cancer rates. Additionally, in migration studies, incidence rates of breast cancer among the descendants of women who moved from low-incidence countries to high-incidence countries converged to those of the high-incidence countries (3–7), which suggests that modifiable lifestyle factors account for much of the global difference in incidence rates. Thus, identification of modifiable risk factors for breast cancer has important implications for breast cancer prevention and risk reduction.
Multiple risk factors for postmenopausal breast cancer have been well established, including height and reproductive factors that are not easily modifiable, such as age at menarche, age at first birth, parity, and age at menopause. However, other established risk factors are more readily modifiable, including greater weight change since age 18 years, menopausal hormone therapy (MHT) use, alcohol consumption, not breastfeeding, and low physical activity level (1, 8).
The population attributable risk percentage (PAR%) is used to estimate the percentage of disease that could be prevented if a risk factor were removed from the population. The PAR% is dependent on the magnitude of the association between the exposure and outcome, as well as the prevalence of the risk factor in the population (9). Therefore, the PAR% may vary across different populations and calendar times, even when the association between the risk factor and breast cancer remains constant. Previous studies in which the PAR% for breast cancer were estimated have been conducted in the United States (10, 11), Europe (12), New Zealand (13), and Australia (14). Previous estimates of the PAR% for modifiable risk factors ranged from 26% in Australia for women 45–69 years of age (14) to 41% in postmenopausal US women (11).
In the United States, the majority of breast cancers are diagnosed among postmenopausal women; recent data have suggested that more than 77% of breast cancer cases in the United States occur among women 50 years of age or older (15). We aimed to determine the proportions of postmenopausal breast cancer in the population that are attributable to established risk factors and to different combinations of risk factors in order to better understand the proportion of breast cancer that could be prevented by changes in modifiable risk factors near menopause (e.g., weight change, physical activity level, and alcohol consumption), as well as risk factors that are considered to be less modifiable (e.g., height, age at first birth, and parity) but that vary between populations. There are accumulating data that the etiology of breast cancer may differ by subtype (16, 17). We therefore assessed whether the PAR% varied by the estrogen receptor (ER) status of the tumor.
METHODS
Study population
The Nurses’ Health Study began in 1976, when 121,700 US female registered nurses between the ages of 30 and 55 years returned an initial questionnaire. Every 2 years, information on reproductive variables, medical history, and lifestyle factors was updated through mailed questionnaires. We used 1980 as the baseline for this analysis because this was the year in which weight at age 18 years was reported. Menopausal status is assessed on each follow-up questionnaire (18) with the question, “Have your periods ceased permanently?” The rate of follow-up for this cohort of women has been greater than 90% through 2010. Voluntary return of the questionnaires was considered to imply informed consent. This study was approved by the Committee on Human Subjects at Brigham and Women's Hospital.
Exposure information
We considered established risk factors for breast cancer as the exposures in the present analysis (19). On the initial questionnaire in 1976, participants reported their height, age at menarche, and reproductive history. Biennially, women reported weight, new pregnancies, MHT use, menopausal status, age at menopause, and diagnoses of benign breast disease. In 1980, weight at age 18 years was reported. In 1986, we assessed total lifetime breastfeeding duration. Women reported family history of breast cancer in 1988, 1992, 1996, 2000, 2004, and 2008. Alcohol consumption was reported every 4 years starting in 1980, and we calculated updated cumulative average alcohol intake up to the assessment just before diagnosis, loss to follow-up, or 2006, which was the year of the last assessment before the end of follow-up (20). Every 2–6 years, we assessed average time per week spent engaging in different types of physical activity; we used this information to calculate metabolic equivalent task hours per week as described previously (21). We calculated updated cumulative average physical activity up to the assessment just before diagnosis, loss to follow-up, or 2006, which was the year of the last assessment before the end of follow-up.
Outcome information
Incident invasive breast cancer cases were identified through self-report on biennial questionnaires until June 1, 2010. Breast cancer diagnoses were confirmed through review of medical records. More than 99% of reported breast cancer cases were confirmed upon review. ER status was obtained from pathology reports.
Deaths
Deaths were identified via next of kin, the post office, or the National Death Index. Physician reviewers blinded to exposure information ascertained causes of death from death certificates, which were supplemented with medical records or interviews with the family or health-care providers if necessary.
Statistical analysis
Person-time for each participant was calculated from the date of return of the 1980 questionnaire for postmenopausal women or the date menopause was first reported to the date of breast cancer diagnosis, any other cancer diagnosis (except nonmelanoma skin cancer), death from any cause, or June 1, 2010, whichever came first. If the date of menopause was never given (4.1% of women), the date the participant became 60 years of age was used in its place because the majority of the cohort reported that menstrual periods had ceased by age 60 years.
We used Cox proportional hazards models to estimate age and multivariable-adjusted relative risks with 95% confidence intervals (22) to facilitate the incorporation of time-varying covariates. For each woman, person-months were allocated to exposure categories beginning at first report of being postmenopausal, and information was updated every 2 years if possible. Multivariable models included family history of breast cancer, personal history of benign breast disease, body mass index (BMI) at age 18 years, weight change since age 18 years, age at menarche, parity by age at first birth, age at menopause, MHT use, physical activity level, history of breastfeeding, height, and alcohol consumption. The missing covariate indicator method, which groups missing observations into a separate category, was used to accommodate missing data (23). In general, the frequency of missing data was low (Web Table 1, available at http://aje.oxfordjournals.org/). The significance of ordinal risk factors was assessed by creating a continuous variable with the median of each of the ordinal variables’ categories assigned as its value and then testing the significance of this continuous variable (24). Given that the effect of parity on breast cancer is conditional on age at first birth (25, 26) and because parity and age at first birth share a common reference group (nulliparous women), we created a variable that cross-classified these 2 exposures. In addition, we examined all potential 2-way interactions between risk factors after grouping categories with similar relative risks to permit a sufficient number of cases in all levels considered. Among all the possible 2-way interactions of the 12 variables considered, only 3 were statistically significant (weight change × age, MHT use × age, and MHT use × BMI). However, given that the magnitudes of the associations with these interactions were small and that the results were materially unchanged, we did not include the interactions in the final presentation. To evaluate the association of exposures by ER status, we performed a cause-specific proportional hazards analysis to estimate separate associations of each exposure with the relative hazard of each type of subtype (27, 28).
The PAR% and 95% confidence intervals were calculated using the methods and %PAR macro developed by Spiegelman et al. (29). This method calculates PAR% by combining the relative risks and the observed prevalence rates of the risk factors of interest. Briefly, the full PAR% was used to quantify the proportional reduction expected in the number of breast cancer cases if all of the risk factors considered were eliminated from the target population. However, we were also interested in evaluating preventive strategies and were specifically interested in the percentage of cases associated with the modifiable risk factors that could be eliminated while keeping other nonmodifiable risk factors unchanged. Therefore, we also presented the partial PAR%, which estimates this percentage of cases that could be eliminated by removing modifiable risk factors only while keeping other risk factors unchanged.
We estimated incidence rates for different combinations of risk factors as a way to provide information about the absolute number of breast cancer cases that could potentially be prevented through changes in these exposures. Multivariable-adjusted absolute incidence rates were estimated using a generalized linear model with the log link function and Poisson working variance, taking into account correlations that were due to repeated measures on the same participants in the estimation of the variance (SAS PROC GENMOD) (30). The reference categories were treated as a single low-risk group, and all others levels combined were considered as a single high-risk category. These rates are to be interpreted as the average rates for study populations with covariate distributions similar to those observed in the Nurses’ Health Study (Web Table 2).
All analyses were conducted using SAS, version 9.2 (SAS Institute, Inc., Cary, North Carolina). A P value <0.05 was used to determine statistical significance, and all tests of statistical significance were 2-sided.
RESULTS
Over the course of follow-up, 8,421 invasive breast cancer cases (5,376 estrogen receptor–positive (ER+) and 1,270 estrogen receptor–negative (ER−)) were documented during 2,424,778 postmenopausal person-years between 1980 and 2010. The majority of women were parous, and among parous women, the mean age at first birth was 25.1 years (Table 1).
Table 1.
Breast Cancer Risk Factors for Postmenopausal Women at Start of Follow-upa (n = 112,951), Nurses’ Health Study, 1980
| Breast Cancer Risk Factor | Frequency, %b |
|---|---|
| Nonmodifiable Risk Factors | |
| Age, yearsc | 47.5 (6.9) |
| Age at menarche, years | |
| ≤12 | 48.6 |
| 13 | 30.7 |
| ≥14 | 20.8 |
| BMI at age 18 yearsd | |
| <19.0 | 17.0 |
| 19.0–20.9 | 33.6 |
| 21.0–22.9 | 29.4 |
| ≥23.0 | 20.0 |
| Height ≥64 inchese | 64.7 |
| Parity and age at first birth | |
| Nulliparous | 6.0 |
| ≥1 child, <25.0 years | 38.2 |
| 1–4 children, 25.0–29.9 years | 30.9 |
| 1–4 children, ≥30.0 years | 9.4 |
| >4 children | 15.4 |
| Benign breast disease history | 42.0 |
| Family history of breast cancer | 12.5 |
| Age at menopause, years | |
| <45.0 | 28.2 |
| 45.0–51.9 | 44.1 |
| ≥52.0 | 27.7 |
| Modifiable Risk Factors | |
| Total breastfeeding durationf, months | |
| None | 40.6 |
| Ever | 59.5 |
| Weight change since age 18 years | |
| Loss to 1.9-kg gain | 17.7 |
| 2.1- to 5.0-kg gain | 10.2 |
| 5.1- to 10.0-kg gain | 17.1 |
| 10.1- to 20.0-kg gain | 28.6 |
| ≥20.1-kg gain | 26.4 |
| Menopausal hormone therapy use | |
| Never or past user | 66.1 |
| Current user | 33.9 |
| Alcohol consumption, g/day | |
| 0 | 23.8 |
| 0.1–4.9 | 42.6 |
| 5.0–15.0 | 21.6 |
| >15.0 | 12.0 |
| Quartile of cumulative average of physical activity, METs/week | |
| 1 | 24.2 |
| 2 | 24.9 |
| 3 | 25.5 |
| 4 | 25.4 |
Abbreviations: BMI, body mass index; METs, metabolic equivalent task hours.
a Either 1980 or the year of the first report of being postmenopausal, whichever was later.
b Values of polytomous variables may not sum to 100% due to rounding.
c Values are expressed as mean (standard deviation).
d Weight (kg)/height (m)2.
e One inch equals 2.54 cm.
f Among parous women.
As expected, earlier age at menarche, lower BMI at age 18 years, greater weight gain since age 18 years, greater alcohol consumption, current use of MHT, and taller height were all significantly positively associated with risk of postmenopausal breast cancer in multivariable models (Table 2). Low physical activity level and never having breastfed were suggestively but not significantly associated with breast cancer risk. Although there were differences in the magnitude of these associations with breast cancer defined by ER status, most risk factors were associated with both ER+ and ER− disease. For example, the association between age at menarche was only slightly stronger for ER+ disease than for ER− disease (age at menarche ≤12 vs. ≥14 years: for ER+ tumors, relative risk (RR) = 1.17, 95% confidence interval (CI): 1.09, 1.26; and for ER− tumors, RR = 1.11, 95% CI: 0.96, 1.29), whereas lower BMI at age 18 years was more strongly associated with ER− breast cancer (for BMI at 18 years <19 vs. ≥23, RR = 1.40, 95% CI: 1.15, 1.70) than with ER+ disease (for BMI at 18 years <19 vs. ≥23, RR = 1.10, 95% CI: 1.00, 1.21). Current MHT use was associated with both ER+ and ER− disease, although the magnitude of association was stronger for ER+ disease (current vs. never or past users: for ER+ tumors, RR = 1.43, 95% CI: 1.34, 1.52; and for ER− tumors, RR = 1.23, 95% CI: 1.08, 1.41).
Table 2.
Relative Risk of Breast Cancer According to Risk Factors Among Postmenopausal Women, Nurses’ Health Study, 1980–2010
| Breast Cancer Risk Factor | Invasive Breast Cancer (8,421 cases and 2,424,778 person-years) | ER+ Invasive Breast Cancer (5,376 cases and 2,421,194 person-years) | ER− Invasive Breast Cancer (1,270 cases and 2,416,662 person-years) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| RRa | 95% CI | P Valueb | RRa | 95% CI | P Valueb | RRa | 95% CI | P Valueb | |
| Nonmodifiable Risk Factors | |||||||||
| Age at menarche, years | <0.0001 | 0.003 | 0.13 | ||||||
| ≤12 | 1.17 | 1.11, 1.24 | 1.17 | 1.09, 1.26 | 1.11 | 0.96, 1.29 | |||
| 13 | 1.09 | 1.03, 1.16 | 1.12 | 1.03, 1.21 | 0.97 | 0.82, 1.14 | |||
| ≥14 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||
| BMI at age 18 yearsc | 0.0001 | 0.01 | 0.002 | ||||||
| <19.0 | 1.16 | 1.08, 1.25 | 1.10 | 1.00, 1.21 | 1.40 | 1.15, 1.70 | |||
| 19.0–20.9 | 1.18 | 1.11, 1.26 | 1.09 | 1.01, 1.19 | 1.46 | 1.22, 1.73 | |||
| 21.0–22.9 | 1.10 | 1.03, 1.18 | 1.05 | 0.97, 1.14 | 1.27 | 1.06, 1.51 | |||
| ≥23.0 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||
| Height, inchesd | <0.0001 | <0.0001 | 0.41 | ||||||
| <63.9 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||
| ≥64.0 | 1.12 | 1.07, 1.17 | 1.15 | 1.08, 1.22 | 1.05 | 0.93, 1.19 | |||
| Parity/age at first birth | <0.0001 | <0.0001 | 0.02 | ||||||
| Nulliparous | 1.23 | 1.12, 1.35 | 1.36 | 1.21, 1.52 | 1.03 | 0.80, 1.32 | |||
| ≥1 child, <25.0 years | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||
| 1–4 children, 25.0–29.9 years | 1.13 | 1.07, 1.19 | 1.13 | 1.06, 1.20 | 1.10 | 0.96, 1.25 | |||
| 1–4 children, ≥30.0 years | 1.34 | 1.24, 1.44 | 1.32 | 1.21, 1.45 | 1.33 | 1.10, 1.61 | |||
| >4 children | 1.06 | 0.96, 1.18 | 1.05 | 0.92, 1.19 | 1.13 | 0.88, 1.46 | |||
| Benign breast disease history | <0.0001 | <0.0001 | <0.0001 | ||||||
| No | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||
| Yes | 1.45 | 1.39, 1.51 | 1.40 | 1.33, 1.48 | 1.66 | 1.48, 1.86 | |||
| Family history of breast cancer | <0.0001 | <0.0001 | <0.0001 | ||||||
| No | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||
| Yes | 1.50 | 1.42, 1.51 | 1.58 | 1.48, 1.69 | 1.37 | 1.18, 1.59 | |||
| Age at menopause, years | <0.0001 | <0.0001 | 0.02 | ||||||
| <45.0 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||
| 45.0–51.9 | 1.24 | 1.17, 1.32 | 1.27 | 1.18, 1.36 | 1.26 | 1.09, 1.45 | |||
| ≥52.0 | 1.43 | 1.34, 1.53 | 1.49 | 1.37, 1.62 | 1.31 | 1.10, 1.55 | |||
| Modifiable Risk Factors | |||||||||
| Breastfeedinge | 0.07 | 0.24 | 0.30 | ||||||
| Never | 1.05 | 1.00, 1.10 | 0.96 | 0.91, 1.02 | 1.07 | 0.94, 1.21 | |||
| Ever | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||
| Weight change since age 18 years, kg | 0.13 | <0.0001 | 0.003 | ||||||
| Loss to 1.9-kg gain | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||
| 2.1- to 5.0-kg gain | 1.12 | 1.02, 1.24 | 1.21 | 1.07, 1.37 | 1.04 | 0.81, 1.33 | |||
| 5.1- to 10.0-kg gain | 1.21 | 1.11, 1.32 | 1.32 | 1.19, 1.47 | 1.16 | 0.94, 1.43 | |||
| 10.1- to 20.0-kg gain | 1.27 | 1.18, 1.37 | 1.37 | 1.25, 1.51 | 1.05 | 0.86, 1.27 | |||
| ≥20.1-kg gain | 1.50 | 1.39, 1.62 | 1.59 | 1.44, 1.75 | 1.38 | 1.13, 1.68 | |||
| Menopausal hormone use | <0.0001 | <0.0001 | 0.002 | ||||||
| Never or past user | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||
| Current user | 1.35 | 1.28, 1.42 | 1.43 | 1.34, 1.52 | 1.23 | 1.08, 1.40 | |||
| Alcohol consumptionf, g/day | <0.0001 | 0.0001 | 0.26 | ||||||
| 0 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||
| 0.1–4.9 | 1.03 | 0.97, 1.09 | 1.02 | 0.94, 1.10 | 1.17 | 1.00, 1.37 | |||
| 5.0–15.0 | 1.13 | 1.05, 1.21 | 1.14 | 1.05, 1.24 | 1.18 | 0.99, 1.41 | |||
| >15.0 | 1.32 | 1.22, 1.42 | 1.37 | 1.25, 1.51 | 1.23 | 1.00, 1.51 | |||
| Quartile of physical activity, METs/week | 0.06 | 0.14 | 0.96 | ||||||
| 1 | 1.07 | 1.01, 1.15 | 1.08 | 0.99, 1.17 | 0.97 | 0.82, 1.15 | |||
| 2 | 1.05 | 0.98, 1.12 | 1.03 | 0.95, 1.11 | 0.96 | 0.82, 1.14 | |||
| 3 | 1.04 | 0.98, 1.11 | 1.05 | 0.97, 1.13 | 1.04 | 0.89, 1.22 | |||
| 4 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||
Abbreviations: CI, confidence interval; ER−, estrogen receptor–negative; ER+ estrogen receptor–positive; METs, metabolic equivalent task hours; RR, relative risk.
a Model was adjusted for age in months, calendar year, and all other risk factors in table.
b The Wald P value is provided for nominal dichotomous variables and the P value for the linear trend is provided for ordinal variables. The P value for age at first birth by parity was obtained using a likelihood ratio test comparing models with and without the construct.
c Weight (kg)/height (m)2.
d One inch equals 2.54 cm.
e Among parous women.
f Cumulative average alcohol consumption.
The full PAR% for models that included age and all modifiable and nonmodifiable risk factors for breast cancer was 92.7% (95% CI: 77.4, 97.8). In models that included all the risk factors assessed in Table 2 (controlling for age but not including age in the partial PAR%), the PAR% was 70.0% (95% CI: 55.0, 80.7) for total invasive breast cancer, 72.5% (95% CI: 54.4, 84.2) for ER+ breast cancer, and 69.5% (95% CI: 29.0, 88.9) for ER− breast cancer (Table 3). Thus, if all women were in the lowest risk category for all of the risk factors considered, the incidence of invasive breast cancer in this population would be reduced by 70%.
Table 3.
Population Attributable Risk for Individual Risk Factors and Combinations of Risk Factors in Postmenopausal Women, Nurses’ Health Study, 1980–2010
| Model or Individual Risk Factor | Invasive Breast Cancer (8,421 cases and 2,424,778 person-years) | ER+ Breast Cancer (5,376 cases and 2,421,194 person-years) | ER− Breast Cancer (1,270 cases and 2,416,662 person-years) | |||
|---|---|---|---|---|---|---|
| PAR% | 95% CI | PAR% | 95% CI | PAR% | 95% CI | |
| Combination of Established Breast Cancer Risk Factors With and Without Age | ||||||
| Model 1a | 92.7 | 77.4, 97.8 | 95.1 | 75.8, 99.1 | 86.8 | 45.9, 97.4 |
| Model 2b | 70.0 | 55.0, 80.7 | 72.5 | 54.4, 84.2 | 69.5 | 29.0, 88.9 |
| Combination of Factors Not Modifiable at Menopause | ||||||
| Model 3c | 55.1 | 43.3, 65.0 | 54.6 | 39.5, 66.9 | 57.0 | 32.2, 74.5 |
| Combination of Factors Modifiable at Menopause | ||||||
| Model 4d | 34.6 | 22.7, 45.4 | 39.7 | 25.8, 51.9 | 27.9 | 0.3, 51.5 |
| Individual Factors Not Modifiable at Menopause | ||||||
| Age | 60.1 | 46.5, 71.0 | 70.4 | 54.5, 81.4 | 31.3 | 8.1, 51.3 |
| Age at menarche | 8.6 | 4.2, 13.0 | 9.8 | 4.3, 15.2 | 5.3 | 0, 10.5 |
| BMIe at age 18 years | 11.0 | 6.0, 15.9 | 6.4 | −0.1, 12.7 | 23.6 | 11.6, 35.0 |
| Height ≥64 inchesf | 6.5 | 3.5, 9.5 | 8.3 | 4.5, 12.1 | 3.0 | −4.9, 10.9 |
| Age at first birth/parity | 9.8 | 6.2, 13.3 | 9.4 | 4.9, 13.8 | 8.7 | −0.1, 17.4 |
| History of BBD | 15.4 | 13.3, 17.6 | 15.0 | 12.2, 17.6 | 19.7 | 14.1, 25.1 |
| Family history of breast cancer | 6.2 | 5.1, 7.3 | 7.3 | 5.9, 8.7 | 4.4 | 2.0, 6.9 |
| Age at menopause | 16.6 | 12.8, 20.3 | 18.4 | 13.8, 23.0 | 14.7 | 5.2, 24.0 |
| Individual Factors Modifiable at Menopause | ||||||
| Breastfeedingg | 1.6 | −0.1, 3.4 | 0 | −2.2, 2.2 | 2.4 | −2.1, 6.8 |
| Weight change since age 18 years | 18.7 | 13.5, 23.7 | 23.9 | 17.6, 30.0 | 10.7 | −2.9, 23.9 |
| Menopausal hormone therapy use | 10.1 | 8.7, 11.6 | 12.2 | 10.4, 13.9 | 7.0 | 2.9, 11.2 |
| Alcohol consumption | 5.9 | 1.2, 10.5 | 7.0 | 1.1, 12.7 | 9.8 | −2.1, 21.4 |
| Physical activity level | 3.3 | −1.0, 7.5 | 2.9 | −2.3, 8.2 | 1.4 | −1.7, 4.5 |
Abbreviations: BBD, benign breast disease; BMI, body mass index; CI, confidence interval; ER−, estrogen receptor–negative; ER+ estrogen receptor–positive; PPAR%, population attributable risk percentage.
a Model 1 is the full model, which includes the following variables: age, age at menarche, BMI at age 18 years, height, parity/age at first birth, history of benign breast disease, family history of breast cancer, age at menopause, breastfeeding, weight change since 18 years, menopausal hormone therapy, alcohol consumption, and physical activity level.
b Model 2 includes all variables in full model except age.
c Model 3 includes the following variables: age at menarche, BMI at age 18 years, height, parity/age at first birth, history of benign breast disease, family history of breast cancer, and age at menopause.
d Model 4 includes the following variables: breastfeeding, weight change since 18 years, menopausal hormone therapy use, alcohol consumption, and physical activity
e Weight (kg)/height (m)2.
f One inch equals 2.54 cm.
g Nulliparous women are considered to have no breastfeeding.
Because many breast cancer risk factors are not considered easily modifiable, we also evaluated more readily modifiable risk factors individually and in combinations (Table 3 and Web Table 2). When considering the modifiable risk factors together as a group, we found that changing one's risk factor profile to the lowest weight gain since age 18 years (i.e., <2-kg weight gain), no alcohol consumption, high physical activity level (i.e., highest quartile), ever breastfeeding, and no current hormone use was associated with a PAR% of 34.6% (95% CI: 22.7, 45.4); the PAR% was higher for ER+ breast cancer (PAR% = 39.7%) than for ER− breast cancer (PAR% = 27.9%). The PAR% for the combination of nonmodifiable factors was 55.1% (95% CI: 43.3, 65.0). The PAR% for different combinations of risk factors are included in Web Table 2. For example, the highest 3-variable PAR% was 31.2% for the combination of weight change since age 18 years, alcohol consumption, and MHT use.
The single modifiable risk factor with the largest PAR% was weight change since age 18 years (for high risk (≥2.1-kg weight gain) vs. low risk (<2.0-kg weight gain), PAR% = 18.7%, 95% CI: 13.5, 23.7). The PAR% was greater for ER+ breast cancer (PAR% = 23.9%, 95% CI: 17.6, 30.0) than for ER− breast cancer (PAR% = 10.7%, 95% CI: −2.9, 23.9). Reproductive risk factors that we did not consider readily modifiable, such as parity and age at first birth combined (PAR% = 9.8%, 95% CI: 6.2, 13.3) and age at menarche (PAR% = 8.6,% 95% CI: 4.2, 13.0) had modest PAR%.
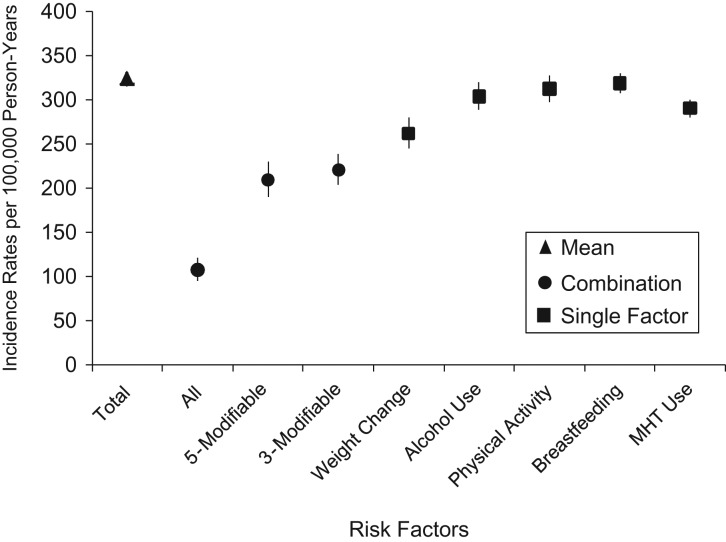
In the present study, the mean incidence rate for invasive breast cancer when considering all risk factors was estimated to be 324 per 100,000 person-years among postmenopausal women (Figure 1 and Web Table 3). This is comparable to incidence rates from US registries, in which the incidence rate for breast cancer among women older than 50 years of age is 354 per 100,000 person-years (31). When all risk factors, both modifiable and nonmodifiable, were set to the lowest risk category, the estimated incidence rate was 108 per 100,000 person-years. If we considered only the modifiable risk factors, we found that changing risk factor profile to the lowest weight gain, no alcohol consumption, high physical activity level, ever breastfeeding, and no hormone use is associated with an estimated incidence rate of 210 per 100,000 person-years. The estimated incidence rates give an approximation of the absolute number of breast cancer cases that could be prevented through changes in these exposures.
Figure 1.
Estimated breast cancer incidence rates with combinations of breast cancer risk factors, Nurses’ Health Study, 1980–2010. Incidence is shown with all variables set to the observed mean (Total); all risk factors combined in Table 2 set to low risk (All); weight change since 18 years of age, alcohol consumption, physical activity level, breastfeeding, and menopausal hormone therapy (MHT) combined and all set to low risk (5-Modifiable); weight change since 18 years of age, alcohol consumption, and MHT combined and all set to low risk (3-Modifiable); and each individual risk factor set to low risk (Weight Change through MHT).
DISCUSSION
In the present study with more than 8,400 cases of invasive breast cancer, we found that 70% of invasive postmenopausal breast cancers could be attributed to known breast cancer risk factors other than age. Although these risk factors could explain a greater extent of ER+ disease, they could also explain the overwhelming majority of ER− disease.
Although the majority of breast cancer risk is attributable to nonmodifiable risk factors, modifiable risk factors together accounted for a large proportion, such that changing the risk factor profile to have the lowest weight gain, no alcohol consumption, high physical activity level, breastfeeding, and no hormone use could reduce the rate of postmenopausal breast cancer by more than 34%. Additionally, the single modifiable factor that had the most substantial influence on breast cancer was weight gain after age 18 years. In our study population, physical activity level and breastfeeding were not significantly associated with risk, and the PAR% for these individual risk factors were minimal. It should be noted that part of the benefit of physical activity is likely to be mediated by weight control, and weight gain was included in multivariable models.
Although the overall PAR% were very similar for ER+ and ER− breast cancers, we did note some differences. Weight change since age 18 years was associated with a larger PAR% for ER+ (23.9%) than for ER− (10.7%) breast cancer. After menopause, adipose tissue serves as the primary source of circulating estrogens; there is a positive linear relationship between postmenopausal BMI and circulating estradiol (32). Interestingly, the findings for BMI earlier in life appear to operate in the opposite direction. For example, being lean at age 18 years was associated with a larger PAR% for ER− breast cancer (23.6%) than for ER+ breast cancer (6.4%). This is consistent with a stronger relative risk reported for ER− breast cancer (33).
We evaluated the role of 12 established breast cancer risk factors individually and in combination in estimating PAR%. In other studies, investigators have evaluated a range of risk factors, and many focused solely on modifiable risk factors. For individual risk factors, our study results are generally similar to those that have been published previously. For example, high alcohol consumption was associated with a PAR% of 6.5% in our study, and others have reported values ranging from 2.8% in Australia (14) to 6.1% in the United States (11). In the present study, weight change since age 18 years was the modifiable risk factor associated with the largest PAR% (18.7%). In other studies evaluating BMI or weight gain since age 18 years, researchers also reported a relatively large PAR% for this risk factor; Wilson et al. (14) reported a PAR% of 12.1% for a BMI greater than 25, and Sprague et al. (11) reported a PAR% of 21% with a weight gain of more than 5 kg since age 18 years. When considering modifiable factors as a group, the PAR% was 34.6%. This is consistent with findings in other studies, in which modifiable risk factors accounted for 26%–40.7% of breast cancers in the populations (11, 12, 14). In the study by Sprague et al. (11), which is most similar to the present study in that it was among postmenopausal US women, the authors reported a PAR% of 40.7% for modifiable risk factors.
Some differences in the individual PARs of risk factors across studies may be due to differences in the prevalence of risk factors, age/menopausal status, and categories of exposures that are considered across populations, as well as the methodologies used to calculate PAR%. For example, in a number of studies, the PAR% for physical activity (range, 3.2%–15.7%) (11, 13, 34, 35) were substantially higher than those observed in our study. This may also reflect true differences in activity levels in the study populations and/or how physical activity was assessed. Additionally, there may be benefits of increasing breastfeeding duration on breast cancer risk that may not be evident in the present study because of the lower range of breastfeeding durations. Prior cohort studies also differed in that some used relative risk (10, 14) and exposure prevalence data (13) from the published literature or were case-control studies (11, 12, 34) and used methods by Bruzzi et al. (36).
The present study has several limitations. We did not consider all potential or proposed risk factors for breast cancer, such as fruit and vegetable consumption for ER− breast cancer. However, we focused on established risk factors (1, 8) that have been consistently demonstrated to be risk factors in other populations. All risk factor information was collected through self-report on questionnaires. Many of these self-reported exposures have been validated. For example, alcohol consumption from food frequency questionnaires has been shown to be highly correlated with 4 weeks of food diaries (r = 0.90) (37). Others have shown that the correlation between average technician-measured weight and self-reported weight was 0.97 (38), and the correlation of physical examinations at the time of entry to college or nursing school with recalled weight at age 18 years was 0.94 (39). Additionally, self-reported exposure information was collected before diagnosis of breast cancer, and therefore misclassification would be nondifferential with respect to the outcome. We restricted the analysis to postmenopausal women. Risk factors for premenopausal and postmenopausal breast cancer may differ; thus, our findings are not generalizable to premenopausal women. However, the majority of breast cancers occur among postmenopausal women. Additionally, our population primarily comprised white women, and our findings may not be generalizable to other racial/ethnic groups. However, although the prevalence of risk factors often differs across population subgroups, many breast cancer risk factors have been documented to operate across racial/ethnic groups, as would be expected from diseases with a common underlying biology (20, 40–52). Although we included family history as a nonmodifiable risk factor, we did not directly test for inherited mutations in genes that increase the risk of breast cancer; these are more common causes of breast cancer in younger, premenopausal women (53). This study also has a number of strengths. This is a large, prospective study with updated exposure information over a long period of follow-up, which reduces misclassification in the estimated relative risks and exposure prevalence rates. We were able to comprehensively evaluate well-established risk factors for breast cancer and additionally evaluate the PAR% for ER+ and ER− disease separately.
In conclusion, established breast cancer risk factors were associated with a large proportion of both ER+ and ER− postmenopausal breast cancers in the population. Additionally, this study provides evidence that more than a third of postmenopausal breast cancers are preventable through changes in modifiable risk factors, providing compelling data that breast cancer is attributable to a number of established risk factors and that a substantial reduction in breast cancer incidence among US women is possible. Changes in the 5 modifiable risk factor profiles could potentially reduce breast cancer incidence rates by 114 cases per 100,000 women-years. Risk factors that we considered not easily modifiable, such as age at menarche, height, and parity/age at first birth, accounted for a substantial PAR%, and because these vary between countries, they may explain a large proportion of the international variation in breast cancer rates. Public health messages highlighting the importance of minimizing weight gain during adult life, little to no alcohol consumption, breastfeeding when possible, and being physically active are important for many chronic diseases, as well as for breast cancer prevention. The most important modifiable risk factor for postmenopausal breast cancer weight gain during adult life; minimizing weight gain is important for prevention.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Channing Division of Network of Medicine, Brigham and Women's Hospital, Boston, Massachusetts (Rulla M. Tamimi, Walter C. Willett, A. Heather Eliassen, David J. Hunter); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Rulla M. Tamimi, Donna Spiegelman, Stephanie A. Smith-Warner, Molin Wang, Mathew Pazaris, Walter C. Willett, A. Heather Eliassen, David J. Hunter); Department of Biostatistics, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Donna Spiegelman, Molin Wang); and Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Stephanie A. Smith-Warner, Walter C. Willett, David J. Hunter).
This work was supported by the National Institutes of Health (grants UM1 CA186107 and P01 CA 87969) and the Breast Cancer Research Foundation.
We thank the staff of the Nurses’ Health Study for their valuable contributions, as well as the following state cancer registries for their help: Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, Washington, Wyoming.
The authors assume full responsibility for analyses and interpretation of these data. The study sponsors were not involved in study design, data collection/analysis/interpretation, or article drafting/submission.
Conflict of interest: none declared.
REFERENCES
- 1.American Cancer Society Breast Cancer Facts & Figures 2013-2014. Atlanta, GA: American Cancer Society, Inc.; 2013. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-042725.pdf. Accessed November 7, 2016. [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, et al. . Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94(2):153–156. [DOI] [PubMed] [Google Scholar]
- 3.Stanford JL, Herrinton LJ, Schwartz SM, et al. . Breast cancer incidence in Asian migrants to the United States and their descendants. Epidemiology. 1995;6(2):181–183. [DOI] [PubMed] [Google Scholar]
- 4.Kolonel LN. Cancer patterns of four ethnic groups in Hawaii. J Natl Cancer Inst. 1980;65(5):1127–1139. [PubMed] [Google Scholar]
- 5.Tominaga S. Cancer incidence in Japanese in Japan, Hawaii, and western United States. Natl Cancer Inst Monogr. 1985;69:83–92. [PubMed] [Google Scholar]
- 6.Yu H, Harris RE, Gao YT, et al. . Comparative epidemiology of cancers of the colon, rectum, prostate and breast in Shanghai, China versus the United States. Int J Epidemiol. 1991;20(1):76–81. [DOI] [PubMed] [Google Scholar]
- 7.Ziegler RG, Hoover RN, Pike MC, et al. . Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993;85(22):1819–1827. [DOI] [PubMed] [Google Scholar]
- 8.Hankinson S, Tamimi RM, Hunter DJ. Breast cancer In: Adami HO, Hunter D, Trichopoulos D, eds. Textbook of Cancer Epidemiology. New York, NY: Oxford University Press; 2008:403–436. [Google Scholar]
- 9.Rothman KJ, Greenland S, eds. Modern Epidemiology. 2nd ed Philadelphia, PA: Lippincot-Raven Publishers; 1998. [Google Scholar]
- 10.Clarke CA, Purdie DM, Glaser SL. Population attributable risk of breast cancer in white women associated with immediately modifiable risk factors. BMC Cancer. 2006;6:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sprague BL, Trentham-Dietz A, Egan KM, et al. . Proportion of invasive breast cancer attributable to risk factors modifiable after menopause. Am J Epidemiol. 2008;168(4):404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnes BB, Steindorf K, Hein R, et al. . Population attributable risk of invasive postmenopausal breast cancer and breast cancer subtypes for modifiable and non-modifiable risk factors. Cancer Epidemiol. 2011;35(4):345–352. [DOI] [PubMed] [Google Scholar]
- 13.Hayes J, Richardson A, Frampton C. Population attributable risks for modifiable lifestyle factors and breast cancer in New Zealand women. Intern Med J. 2013;43(11):1198–1204. [DOI] [PubMed] [Google Scholar]
- 14.Wilson LF, Page AN, Dunn NA, et al. . Population attributable risk of modifiable risk factors associated with invasive breast cancer in women aged 45-69 years in Queensland, Australia. Maturitas. 2013;76(4):370–376. [DOI] [PubMed] [Google Scholar]
- 15.Sineshaw HM, Gaudet M, Ward EM, et al. . Association of race/ethnicity, socioeconomic status, and breast cancer subtypes in the National Cancer Data Base (2010-2011). Breast Cancer Res Treat. 2014;145(3):753–763. [DOI] [PubMed] [Google Scholar]
- 16.Barnard ME, Boeke CE, Tamimi RM. Established breast cancer risk factors and risk of intrinsic tumor subtypes. Biochim Biophys Acta. 2015;1856(1):73–85. [DOI] [PubMed] [Google Scholar]
- 17.Yang XR, Chang-Claude J, Goode EL, et al. . Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. J Natl Cancer Inst. 2011;103(3):250–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Nurses’ Health Study Questionnaires. http://www.nurseshealthstudy.org/participants/questionnaires. Accessed November 7, 2016.
- 19.Harris JR, Lippman ME, Veronesi U, et al. . Breast cancer (1). N Engl J Med. 1992;327(5):319–328. [DOI] [PubMed] [Google Scholar]
- 20.Fuchs CS, Stampfer MJ, Colditz GA, et al. . Alcohol consumption and mortality among women. N Engl J Med. 1995;332(19):1245–1250. [DOI] [PubMed] [Google Scholar]
- 21.Eliassen AH, Hankinson SE, Rosner B, et al. . Physical activity and risk of breast cancer among postmenopausal women. Arch Intern Med. 2010;170(19):1758–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersen PK, Gill RD. Cox's regression model for counting processes: a large sample study. Ann Statist. 1982;10(4):1100–1120. [Google Scholar]
- 23.Miettinen OS. Theoretical Epidemiology: Principles of Occurrence Research in Medicine. New York, NY: John Wiley; 1985. [Google Scholar]
- 24.Greenland S. Avoiding power loss associated with categorization and ordinal scores in dose-response and trend analysis. Epidemiology. 1995;6(4):450–454. [DOI] [PubMed] [Google Scholar]
- 25.Trichopoulos D, Hsieh CC, MacMahon B, et al. . Age at any birth and breast cancer risk. Int J Cancer. 1983;31(6):701–704. [DOI] [PubMed] [Google Scholar]
- 26.Rosner B, Colditz GA, Willett WC. Reproductive risk factors in a prospective study of breast cancer: the Nurses’ Health Study. Am J Epidemiol. 1994;139(8):819–835. [DOI] [PubMed] [Google Scholar]
- 27.Prentice RL, Kalbfleisch JD, Peterson AV Jr, et al. . The analysis of failure times in the presence of competing risks. Biometrics. 1978;34(4):541–554. [PubMed] [Google Scholar]
- 28.Kalbfleisch JD, Prentice RL. The Statistical Anlaysis of Failure Time Data. 2nd ed New York, NY: John Wiley & Sons, Inc; 2002. [Google Scholar]
- 29.Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risks in cohort studies: examples and software. Cancer Causes Control. 2007;18(5):571–579. [DOI] [PubMed] [Google Scholar]
- 30.D'Agostino RB, Lee ML, Belanger AJ, et al. . Relation of pooled logistic regression to time dependent Cox regression analysis: the Framingham Heart Study. Stat Med. 1990;9(12):1501–1515. [DOI] [PubMed] [Google Scholar]
- 31.Surveillance Epidemiology and End Results (SEER) Program, Division of Cancer Control and Population Sciences SEER*Stat Database: Populations - Total US (1969-2013). Washington, DC: National Cancer Institute; 2014. http://seer.cancer.gov/. [Google Scholar]
- 32.Key TJ, Appleby PN, Reeves GK, et al. . Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95(16):1218–1226. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki R, Iwasaki M, Inoue M, et al. . Body weight at age 20 years, subsequent weight change and breast cancer risk defined by estrogen and progesterone receptor status–the Japan public health center-based prospective study. Int J Cancer. 2011;129(5):1214–1224. [DOI] [PubMed] [Google Scholar]
- 34.Mezzetti M, La Vecchia C, Decarli A, et al. . Population attributable risk for breast cancer: diet, nutrition, and physical exercise. J Natl Cancer Inst. 1998;90(5):389–394. [DOI] [PubMed] [Google Scholar]
- 35.Barnes BB, Steindorf K, Hein R, et al. . Population attributable risk of invasive postmenopausal breast cancer and breast cancer subtypes for modifiable and non-modifiable risk factors. Cancer Epidemiol. 2011;35(4):345–352. [DOI] [PubMed] [Google Scholar]
- 36.Bruzzi P, Green SB, Byar DP, et al. . Estimating the population attributable risk for multiple risk factors using case-control data. Am J Epidemiol. 1985;122(5):904–914. [DOI] [PubMed] [Google Scholar]
- 37.Giovannucci E, Colditz G, Stampfer MJ, et al. . The assessment of alcohol consumption by a simple self-administered questionnaire. Am J Epidemiol. 1991;133(8):810–817. [DOI] [PubMed] [Google Scholar]
- 38.Rimm EB, Stampfer MJ, Colditz GA, et al. . Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1(6):466–473. [DOI] [PubMed] [Google Scholar]
- 39.Troy LM, Hunter DJ, Manson JE, et al. . The validity of recalled weight among younger women. Int J Obes Relat Metab Disord. 1995;19(8):570–572. [PubMed] [Google Scholar]
- 40.Adams-Campbell LL, Rosenberg L, Rao RS, et al. . Strenuous physical activity and breast cancer risk in African-American women. J Natl Med Assoc. 2001;93(7–8):267–275. [PMC free article] [PubMed] [Google Scholar]
- 41.Colditz GA, Willett WC, Hunter DJ, et al. . Family history, age, and risk of breast cancer. Prospective data from the Nurses’ Health Study. JAMA. 1993;270(3):338–343. [PubMed] [Google Scholar]
- 42.Palmer JR, Boggs DA, Adams-Campbell LL, et al. . Family history of cancer and risk of breast cancer in the Black Women's Health Study. Cancer Causes Control. 2009;20(9):1733–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kilfoy BA, Zhang Y, Shu XO, et al. . Family history of malignancies and risk of breast cancer: prospective data from the Shanghai women's health study. Cancer Causes Control. 2008;19(10):1139–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hunter DJ, Colditz GA, Hankinson SE, et al. . Oral contraceptive use and breast cancer: a prospective study of young women. Cancer Epidemiol Biomarkers Prev. 2010;19(10):2496–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenberg L, Palmer JR, Wise LA, et al. . A prospective study of female hormone use and breast cancer among black women. Arch Intern Med. 2006;166(7):760–765. [DOI] [PubMed] [Google Scholar]
- 46.Colditz GA, Hankinson SE, Hunter DJ, et al. . The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med. 1995;332(24):1589–1593. [DOI] [PubMed] [Google Scholar]
- 47.Rosenberg L, Boggs DA, Wise LA, et al. . Oral contraceptive use and estrogen/progesterone receptor-negative breast cancer among African American women. Cancer Epidemiol Biomarkers Prev. 2010;19(8):2073–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fung TT, Hu FB, McCullough ML, et al. . Diet quality is associated with the risk of estrogen receptor-negative breast cancer in postmenopausal women. J Nutr. 2006;136(2):466–472. [DOI] [PubMed] [Google Scholar]
- 49.Boggs DA, Palmer JR, Wise LA, et al. . Fruit and vegetable intake in relation to risk of breast cancer in the Black Women's Health Study. Am J Epidemiol. 2010;172(11):1268–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agurs-Collins T, Rosenberg L, Makambi K, et al. . Dietary patterns and breast cancer risk in women participating in the Black Women's Health Study. Am J Clin Nutr. 2009;90(3):621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boggs DA, Palmer JR, Stampfer MJ, et al. . Tea and coffee intake in relation to risk of breast cancer in the Black Women's Health Study. Cancer Causes Control. 2010;21(11):1941–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ganmaa D, Willett WC, Li TY, et al. . Coffee, tea, caffeine and risk of breast cancer: a 22-year follow-up. Int J Cancer. 2008;122(9):2071–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Friebel TM, Domchek SM, Rebbeck TR. Modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.