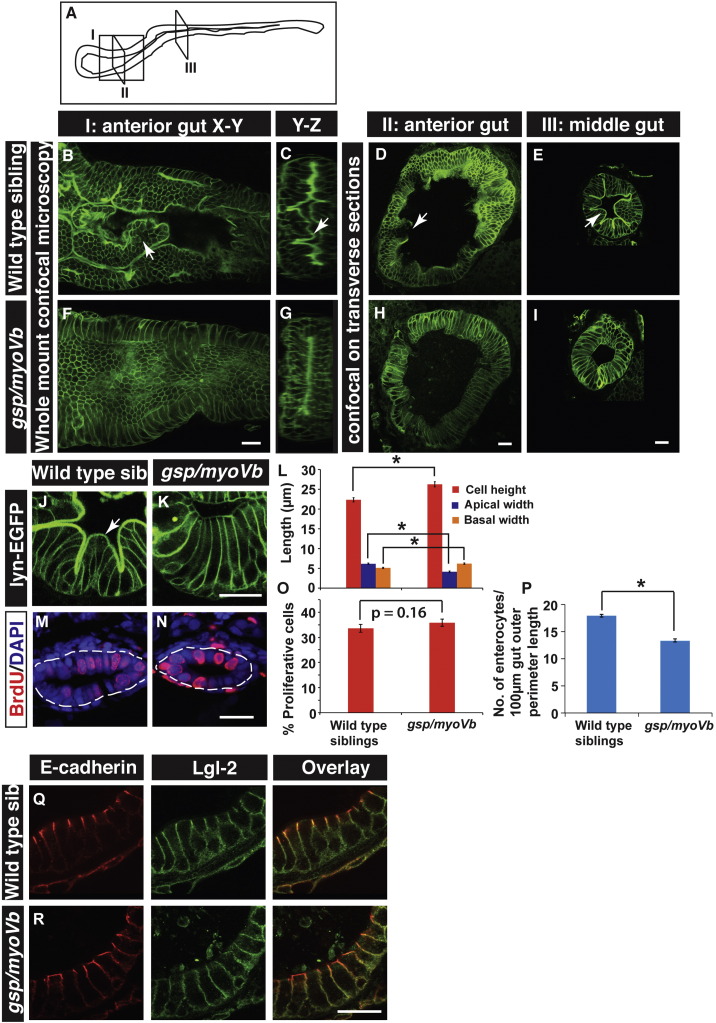
Fig. 3.
The loss of intestinal folds in gsp/myoVb mutants is accompanied by changes in cell shapes.
A schematic showing various regions of the zebrafish larval gut (A). The boxed regions indicate the parts of the intestine where whole mounts were imaged (I) or intestines were sectioned (II, III). Confocal images of the whole mount (B, F), orthogonal sections (C, G), immuno-histological sections (D, E, H, I, J, K) of wild type sibling (B, C, D, E, J) and gsp/myoVb mutant (F, G, H, I, K) intestines stained for lyn-EGFP at 6dpf. Note the absence of intestinal folds in the gsp/myoVb mutants. The whole mount analysis was done on 4 siblings and 3 mutants whereas the immunohistology analysis was done on 9 siblings and 7 mutants. Bar graph (L) showing measurements of the height, apical width and basal width of enterocytes in wild type and gsp/myoVb mutant larvae at 6dpf. The enterocytes are taller and have a narrower apical domain in the gsp/myoVb mutants as compared to wild type. BrdU staining in wild type (M) and gsp/myoVb mutant larvae (N) at 3dpf followed by estimation of proliferation indices, which are represented in a bar graph (O). Number of enterocytes per 100 μm gut perimeter length was estimated from sections for 6-day-old wild type and gsp/myoVb mutant larvae and shown in a bar graph (P). Immuno-histological sections of zebrafish intestine in the wild type (Q) and gsp/myoVb mutant larvae (R) stained using anti E-cadherin and anti-Lgl2 antibodies. Arrows in B–E and J point to the intestinal folds. The square brackets show the comparison and asterisks indicate the statistically significant difference at p < 0.05 by Student's t-test. The error bars in L, O, and P represent SEM. Scale bars are equivalent to 20 μm. Abbreviation: sib = sibling.