Abstract
Background
Feeding tube placement is common among patients undergoing gastrectomy, and national guidelines currently recommend consideration of a feeding jejunostomy tube (FJT) for all patients undergoing resection for gastric cancer. However, data are limited regarding the safety of FJT placement at the time of gastrectomy for gastric cancer.
Methods
The 2005–2011 American College of Surgeons National Surgical Quality Improvement Program Participant User Files were queried to identify patients who underwent gastrectomy for gastric cancer. Subjects were classified by the concomitant placement of an FJT. Groups were then propensity matched using a 1:1 nearest neighbor algorithm, and outcomes were compared between groups. The primary outcomes of interest were overall 30-d overall complications and mortality. Secondary end points included major complications, surgical site infection, and early reoperation.
Results
In total, 2980 subjects underwent gastrectomy for gastric cancer, among whom 715 (24%) also had an FJT placed. Patients who had an FJT placed were more likely to be male (61.6% versus 56.6%, P = 0.02), have recent weight loss (21.0% versus 14.8%, P < 0.01), and have undergone recent chemotherapy (7.9% versus 4.2%, P < 0.01) and radiation therapy (4.2% versus 1.3%, P < 0.01). They were also more likely to have undergone total (compared with partial) gastrectomy (66.6% versus 28.6%, P < 0.01) and have concomitant resection of an adjacent organ (40.4 versus 24.1%, P < 0.01). After adjustment with propensity matching, however, all baseline characteristics and treatment variables were highly similar. Between groups, there were no statistically significant differences in 30-d overall complications (38.8% versus 36.1%, P =0.32) or mortality (5.8 versus 3.7%, P =0.08). There were also no differences in major complications, surgical site infection, or early reoperation. Operative time was slightly longer among patients with feeding tubes placed (median, 248 versus 233 min, P = 0.01), but otherwise there were no significant differences in any outcomes between groups.
Conclusions
Concomitant placement of FJT at the time of gastrectomy may result in slightly increased operative times but does not appear to lead to increased perioperative morbidity or mortality. Further investigation is needed to identify the patients most likely to benefit from FJT placement.
Keywords: Gastric cancer, Gastrectomy, Feeding jejunostomy tube, Nutrition, Outcomes
1. Introduction
Gastric cancer accounts for 12% of cancer-related deaths worldwide and remains the second leading cause of death after lung cancer [1]. In the multimodality treatment of gastric cancer, adjuvant or perioperative therapy has been shown in randomized trials to improve survival [2–4]. However, as highlighted by the Medical Research Council Adjuvant Gastric Cancer Infusional Chemotherapy (MAGIC) trial, less than one-third of patients are able to tolerate and complete adjuvant therapy after gastrectomy [2]. Common gastrointestinal toxicities encountered during administration of chemotherapy include anorexia, nausea, vomiting, and diarrhea, all of which may increase risk of failure to complete adjuvant therapy [5].
The placement of a feeding jejunostomy tube (FJT) at the time of gastrectomy offers alternative and supplementary nutritional access, which may serve to maintain caloric requirements even in the setting of the profound gastrointestinal toxicity that is commonly associated with adjuvant therapy. Because an FJT may thus theoretically permit a patient suffering from the side effects of systemic therapy to complete treatment, the National Comprehensive Cancer Network currently recommends that FJT placement to be considered for select patients who will be receiving postoperative adjuvant therapy [6]. However, in other gastrointestinal oncologic operations, such as pancreaticoduodenectomy for pancreatic cancer, FJT placement has been associated with increased perioperative morbidity [7,8]. Because the hypothetical benefits of FJT placement remain unproven—and there are limited data regarding the effect of FJT placement on short-term peri-operative outcomes—it is important to evaluate whether placement of an FJT results in inferior perioperative outcomes among patients undergoing gastrectomy for gastric cancer [9].
The American College of Surgeons National Surgical Quality Improvement Program (NSQIP) provides the largest, risk-adjusted, validated data set of 30-d surgical outcomes of the United States. Because data in NSQIP are collected from a variety of hospitals across the United States, it is useful for analyzing perioperative outcomes that may then be generalizable across institutions. In this study, we hypothesize that concomitant FJT placement in patients undergoing gastrectomy for gastric cancer does not affect short-term perioperative outcomes.
2. Materials and methods
The Duke University Institutional Review Board approved this retrospective analysis. The NSQIP Participant User Files for 2005–2011 were queried to identify patients who had undergone gastrectomy for gastric cancer. Patients with International Classification of Diseases Ninth Revision codes 151.0–151.9 (primary malignancy of the stomach) were identified and cross-referenced with current procedural terminology (CPT) codes: 43631/43632/43633/43634 (partial gastrectomy) and 43620/43621/43622 (total gastrectomy). Exclusion criteria included subjects that had an associated CPT code that was inconsistent with a primary diagnosis of gastric cancer, had undergone emergent surgery, or had disseminated malignancy. Subjects were then classified by the concomitant placement of an FJT (identified by CPT codes 44300 and 44015). Baseline characteristics and outcomes were compared between groups using Pearson chi-square test for categorical variables and Student t-test for continuous variables.
To adjust for nonrandom differences between groups, we developed propensity scores, defined as the conditional probability of undergoing concomitant FJT placement. Propensity scores were based on the following variables: age, sex, body mass index, diabetes mellitus, chronic obstructive pulmonary disease, coronary artery disease, bleeding disorders, dyspnea, functional status, American Society of Anesthesiologists classification, existing do not resuscitate order, tobacco use, alcohol use >2 drinks per day, recent steroid use, year of operation, preoperative chemotherapy, preoperative radiation therapy, extent of resection (total versus subtotal gastrectomy), and concomitant major organ resection. Patients were then matched on these scores using a 1:1 nearest neighbor algorithm. Our primary outcomes of interest were 30-d overall complications and mortality. Secondary end points included major complications, surgical site infection, and early reoperation.
Missing data in the NSQIP database were handled using complete case analysis. We made an affirmative decision to control for type 1 error at the level of the comparison. For comparison of baseline characteristics and outcomes, P values <0.05 were used to indicate statistical significance. Standardized differences were used to compare baseline characteristics and treatment variables between propensity-matched groups, with standardized differences <0.20 representing a negligible difference for each covariate. All statistical analyses were performed using R (The R Foundation for Statistical Computing, version 3.0.2, Vienna, Austria).
3. Results
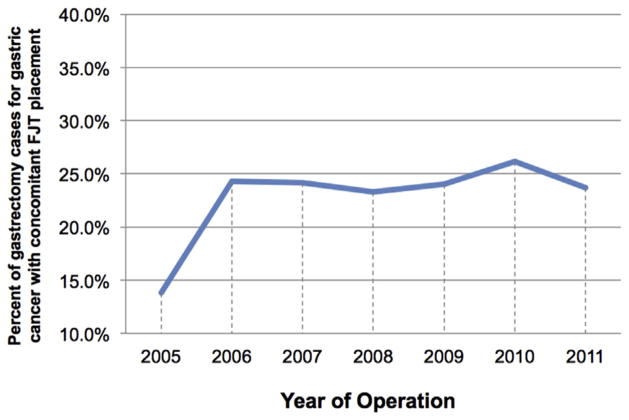
In total, 5881 patients were identified who had a primary diagnosis of gastric cancer. Of these, 2658 were excluded based on CPT codes that were inconsistent with a primary procedure of gastrectomy and 243 because of emergent surgery or disseminated cancer. This resulted in the study population of 2980 subjects. Of these patients, 715 (24%) had an FJT placed at the time of gastrectomy. Over the study period, there was no statistically significant change in the rate of FJT placement (Figure).
Figure.
Trends in utilization of FJTs with gastrectomy for gastric cancer from 2005–2011. (Color version of the figure is available online.)
Subjects who met inclusion criteria were then grouped based on the concomitant placement of an FJT. Patients who had an FJT placedatthetimeofgastrectomyweremore likelytobemale (61.6% versus 56.6%, P = 0.02), have recent weight loss (21.0% versus 14.8%, P < 0.01), and have undergone recent chemotherapy (7.9% versus 4.2%, P < 0.01) and radiation therapy (4.2 versus 1.3%, P < 0.01). They were also more likely to have undergone total (compared with partial) gastrectomy (66.6% versus 28.6%, P < 0.01) and have concomitant resection of an adjacent organ (40.4% versus 24.1%, P < 0.01; Table 1). However, after adjustment with propensity matching, all baseline and treatment variables were highly similar between groups (Table 2).
Table 1.
Baseline characteristics of patients undergoing gastrectomy, stratified by FJT use.
| Variable | Overall, n = 2980 | No FJT, n = 2265 | FJT, n = 715 | P value |
|---|---|---|---|---|
| Age in years, median (IQR) | 68 (58–77) | 68 (58–77) | 69 (57–77) | 0.43 |
| Female sex | 1256 (42.2) | 982 (43.4) | 274 (38.4) | 0.02 |
| ASA class ≥3 | 2003 (67.4) | 1502 (66.4) | 501 (70.3) | 0.06 |
| BMI (kg/m2), median (IQR) | 25.7 (22.6–29.4) | 25.7 (22.7–29.4) | 25.1 (22.5–29.2) | 0.11 |
| Preoperative sepsis | 0.63 | |||
| None | 2900 (98.1) | 2204 (98) | 696 (98.6) | |
| SIRS | 46 (1.6) | 37 (1.6) | 9 (1.3) | |
| Sepsis | 9 (0.3) | 8 (0.4) | 1 (0.1) | |
| Septic shock | 0 (0) | 0 (0) | 0 (0) | |
| Dyspnea | 366 (12.3) | 292 (12.9) | 74 (10.3) | 0.08 |
| Nonindependent functional status | 154 (5.2) | 123 (5.4) | 31 (4.3) | 0.29 |
| DNR status | 11 (0.4) | 11 (0.5) | 0 (0) | 0.08 |
| Tobacco use | 543 (18.2) | 421 (18.6) | 122 (17.1) | 0.39 |
| Alcohol use | 92 (3.3) | 71 (3.4) | 21 (3.1) | 0.87 |
| Diabetes | 562 (18.9) | 419 (18.5) | 143 (20) | 0.40 |
| COPD | 166 (5.6) | 125 (5.5) | 41 (5.7) | 0.90 |
| Coronary artery disease | 397 (14.3) | 294 (14) | 103 (15.4) | 0.39 |
| Dialysis dependence | 16 (0.5) | 15 (0.7) | 1 (0.1) | 0.14 |
| Bleeding disorder | 115 (3.9) | 88 (3.9) | 27 (3.8) | 0.98 |
| Recent steroid use | 55 (1.8) | 42 (1.9) | 13 (1.8) | 0.99 |
| Recent weight loss | 486 (16.3) | 336 (14.8) | 150 (21) | <0.01 |
| Preoperative chemotherapy | 142 (5.1) | 89 (4.2) | 53 (7.9) | <0.01 |
| Preoperative radiation therapy | 55 (2) | 27 (1.3) | 28 (4.2) | <0.01 |
| Preoperative serum albumin | 0.16 | |||
| Abnormal (≤3.5 g/dL) | 634 (21.3) | 482 (21.3) | 152 (21.3) | |
| Missing | 640 (21.5) | 504 (22.3) | 136 (19) | |
| Normal | 1706 (57.2) | 1279 (56.5) | 427 (59.7) | |
| Contaminated/dirty case | 112 (3.8) | 84 (3.7) | 28 (3.9) | 0.89 |
| Extent of gastrectomy | <0.01 | |||
| Partial gastrectomy | 1856 (62.3) | 1617 (71.4) | 239 (33.4) | |
| Total gastrectomy | 1124 (37.7) | 648 (28.6) | 476 (66.6) | |
| Concomitant resection of adjacent organ | 834 (28) | 545 (24.1) | 289 (40.4) | <0.01 |
ASA = American Society of Anesthesiologists; BMI = body mass index; COPD = chronic obstructive pulmonary disease; DNR = do not resuscitate; IQR = interquartile range; SIRS = systemic inflammatory response syndrome.
Data are represented as counts (percentages) unless otherwise stated.
Table 2.
Adjusted baseline characteristics of patients undergoing gastrectomy, stratified by FJT use.
| Variable | No FJT, n = 704 | FJT, n = 704 | Standardized differences, % |
|---|---|---|---|
| Age in years, median (IQR) | 68.5 (57–76) | 69 (57–77) | 1 |
| Female sex | 267 (37.9) | 268 (38.1) | 0 |
| ASA class ≥3 | 499 (70.9) | 494 (70.2) | 2 |
| BMI (kg/m2), median (IQR) | 25.5 (22.4–29.3) | 25.2 (22.5–29.2) | 2 |
| Preoperative sepsis | 7 | ||
| None | 692 (98.3) | 695 (98.7) | |
| SIRS | 8 (1.1) | 8 (1.1) | |
| Sepsis | 4 (0.6) | 1 (0.1) | |
| Septic shock | 0 (0) | 0 (0) | |
| Dyspnea | 83 (11.8) | 73 (10.4) | 5 |
| Nonindependent functional status | 34 (4.8) | 31 (4.4) | 2 |
| DNR status | 0 (0) | 0 (0) | 0 |
| Tobacco use | 117 (16.6) | 121 (17.2) | 2 |
| Alcohol use | 20 (3.1) | 21 (3.2) | 1 |
| Diabetes | 142 (20.2) | 142 (20.2) | 0 |
| COPD | 45 (6.4) | 39 (5.5) | 4 |
| CAD | 94 (14.5) | 103 (15.7) | 3 |
| Dialysis dependence | 3 (0.4) | 1 (0.1) | 5 |
| Bleeding disorder | 28 (4) | 26 (3.7) | 2 |
| Recent steroid use | 12 (1.7) | 13 (1.8) | 1 |
| Recent weight loss | 146 (20.7) | 148 (21) | 1 |
| Preoperative chemotherapy | 42 (6.5) | 52 (7.9) | 6 |
| Preoperative radiation therapy | 20 (3.1) | 28 (4.3) | 6 |
| Preoperative serum albumin | 7 | ||
| Abnormal (≤3.5 g/dL) | 165 (23.4) | 151 (21.4) | |
| Missing | 136 (19.3) | 133 (18.9) | |
| Normal | 403 (57.2) | 420 (59.7) | |
| Contaminated/dirty case | 32 (4.5) | 26 (3.7) | 4 |
| Extent of resection | 0 | ||
| Partial gastrectomy | 237 (33.7) | 238 (33.8) | |
| Total gastrectomy | 467 (66.3) | 466 (66.2) | |
| Concomitant resection of adjacent organ | 273 (38.8) | 282 (40.1) | 3 |
ASA = American Society of Anesthesiologists; BMI = body mass index; CAD = coronary artery disease; COPD = chronic obstructive pulmonary disease; DNR = do not resuscitate; IQR = interquartile range; SIRS = systemic inflammatory response syndrome.
Data are represented as counts (percentages) unless otherwise stated. Standardized differences <20% represent negligible differences between groups for a particular covariate.
Adjusted outcomes are shown in Table 3. Between groups, there were no statistically significant differences in 30-d overall complications (38.8% versus 36.1%, P = 0.32) or 30-d mortality (5.8% versus 3.7%, P = 0.08). Although operative time was slightly longer in the FJT group (median, 248 versus 233 min, P < 0.01), there were no corresponding differences in any of our secondary outcomes, which included major complications, surgical site infection, and early reoperation.
Table 3.
Adjusted univariable outcomes of patients undergoing gastrectomy after adjustment with propensity matching, stratified by FJT use.
| Variable | No FJT, n = 704 | FJT, n = 704 | P value |
|---|---|---|---|
| 30-d mortality | 26 (3.7) | 41 (5.8) | 0.08 |
| Overall complication rate | 254 (36.1) | 273 (38.8) | 0.32 |
| Major complication rate | 212 (30.1) | 217 (30.8) | 0.82 |
| Early return to the OR | 65 (9.2) | 65 (9.2) | 0.99 |
| Length of stay in days, median (IQR) | 9 (7–15) | 10 (8–14) | 0.95 |
| Operative time in minutes, median (IQR) | 233 (170–299) | 248 (194–306) | <0.01 |
| Superficial SSI | 34 (4.8) | 50 (7.1) | 0.09 |
| Deep SSI | 13 (1.8) | 10 (1.4) | 0.67 |
| Organ space SSI | 62 (8.8) | 59 (8.4) | 0.85 |
| Wound dehiscence | 13 (1.8) | 10 (1.4) | 0.67 |
| Sepsis | 57 (8.1) | 63 (8.9) | 0.63 |
| Septic shock | 35 (5) | 52 (7.4) | 0.07 |
| Pneumonia | 61 (8.7) | 69 (9.8) | 0.52 |
| Reintubation | 44 (6.2) | 52 (7.4) | 0.46 |
| Prolonged (>48 h) ventilator dependence | 48 (6.8) | 51 (7.2) | 0.84 |
| Pulmonary embolism | 10 (1.4) | 9 (1.3) | 0.99 |
| Acute kidney injury | 3 (0.4) | 6 (0.9) | 0.51 |
| Renal failure | 8 (1.1) | 3 (0.4) | 0.23 |
| UTI | 24 (3.4) | 45 (6.4) | 0.01 |
| Stroke | 4 (0.6) | 4 (0.6) | 0.99 |
| Coma | 0 (0) | 5 (0.7) | 0.06 |
| Cardiac arrest | 10 (1.4) | 15 (2.1) | 0.42 |
| Myocardial infarction | 12 (1.7) | 10 (1.4) | 0.83 |
| Postoperative bleeding | 64 (9.1) | 55 (7.8) | 0.44 |
| Deep venous thrombosis | 16 (2.3) | 11 (1.6) | 0.44 |
IQR = interquartile range; OR = operating room; SSI = surgical site infection.
Data are represented as counts (percentages) unless otherwise stated.
4. Discussion
Gastrectomy for gastric cancer remains a potentially highly morbid procedure, with complications occurring in nearly one-quarter of cases [10]. Patients undergoing treatment for gastric cancer often fail to meet caloric requirements via an oral diet, either due to the nature of their disease (obstruction) or because they are prohibited from oral intake by clinicians in the postoperative setting. As a result, postoperative malnutrition is highly prevalent in this population [11]. Moreover, not only does malnutrition affect surgical outcomes it may also significantly reduce the number of patients successfully progressing to or completing adjuvant therapy, which has become a critical component of the multimodality treatment of gastric cancer [2–4]. Adjuvant therapy trials such as Intergroup Study 0116 and perioperative therapy trials such as MAGIC have clearly shown survival benefit with the addition of multimodality therapy [2,4]. However, these trials also demonstrated that a large number of patients are unable to complete—or even commence—planned adjuvant therapy. For example, a caloric intake of at least 1500 kcal per day was required by the Intergroup trial as an eligibility criteria, demonstrating the importance of adequate nutrition to proceed with adjuvant treatment [4]. In the MAGIC trial, only 66% of patients were able to successfully initiate adjuvant therapy, and even fewer (42%) were able to complete the full planned regimen [2]. Similarly, 36% of patients in the Intergroup trial were unable to complete postoperative therapy, mostly due to the toxic side effects of treatment [4]. To counteract this issue, consideration of FJT placement at the time of gastrectomy has been advocated, both to optimize postoperative nutrition and also to maintain enteral access (and thus caloric requirements) during the potentially profound gastrointestinal toxicity that may occur during adjuvant therapy. The National Comprehensive Cancer Network guidelines currently recommend consideration for FJT placement with the goal of increasing the use and completion rate of adjuvant therapy [6].
Although this hypothetical benefit of FJT placement remains unproven, the practice is common during gastrectomy, with rates of concomitant feeding tube placement stable over time at approximately 25% of cases. Despite the frequent placement of FJT during gastrectomy, the effect of this practice on short-term postoperative outcomes has not been well established. Only a single group, Patel et al. in 2013, has addressed this concern, comparing outcomes among 132 total patients who underwent gastrectomy either with or without FJT placement for gastric cancer (66 with concomitant FJT placement and 66 without). The authors found that FJT was not associated with an improved rate of successful adjuvant therapy and in fact noted that FJT placement was highly associated with an increased rate of postoperative morbidity (hazard ratio, 4.8, P = 0.02) [9]. However, this study was limited by reporting only the relatively small experience of a single institution. Yet, these findings remain the only published data regarding the safety profile specific to concomitant FJT placement at the time of gastrectomy in gastric cancer patients. Therefore, our goal in this study was to use the NSQIP database, which contains aggregated data from both academic and community centers across the United States, to specifically address the question of whether FJT placement at the time of gastrectomy for gastric cancer affects short-term, nononcologic perioperative outcomes.
With 2980 included patients, this study represents the largest analysis of perioperative outcomes in patients undergoing gastrectomy for gastric cancer with or without FJT placement. After adjustment with propensity matching, all baseline patient characteristics between the FJT and no-FJT groups were highly similar (Table 2). We found no statistically significant differences in 30-d overall complications or mortality rates between groups (Table 3). Moreover, although operative times were slightly longer in the FJT group (248 versus 233 min, P < 0.01), we did not observe any corresponding increase in the rates of major complications, surgical site infection, or early reoperation. Patients in the FJT group did have slightly higher rates of urinary tract infections (UTI; 6.4% versus 3.4%, P = 0.01); however, the clinical significance of this finding is unclear. Although it is possible that longer operative times contributed to a higher rate of UTI, nearly all patients likely left the operating room with a urinary catheter in place. NSQIP does not provide granular data on the length of catheter placement, but one possible explanation is that patients who underwent FJT placement also had a longer duration of urinary catheterization, possibly to monitor fluid balance in the setting of initiation and maintenance of tube feeds. Regardless of the reason, it is highly unlikely that placement of the feeding tube itself was related to the development of UTIs, particularly in the absence of any other increase in perioperative complications.
Notably, in other gastrointestinal oncologic surgeries that commonly use feeding tubes for enteral nutrition, concomitant FJT placement has been associated with inferior outcomes. Padussis et al. [8] evaluated FJT placement in patients undergoing the Whipple procedure for pancreatic cancer. In their series of 4930 patients, FJT placement was associated with an 11% increase in overall morbidity, specifically in rates of deep surgical site infection, pneumonia, unplanned reintubation, acute renal failure, and sepsis. Similarly, in institutional series evaluating FJT placement at the time of esophagectomy, the FJT groups consistently demonstrated increases in infectious and other complications [12–14]. These findings are in contrast to this present study, which did not find a difference in postoperative outcomes between patients who underwent gastrectomy with or without concomitant FJT placement.
One substantial difference between gastrectomy and esophagectomy and/or pancreaticoduodenectomy, however, is the rate of anastomotic leak after surgery. Gastrectomy has reported leak rates of approximately 1%, compared with approximately 12% and 13% for esophagectomy and pancreaticoduodenectomy, respectively [15–17]. The presence of intra-abdominal contamination—particularly alongside corrosive pancreatic fluid—may significantly increase the risk of infection related to an indwelling foreign body, especially in the immediate period after FJT placement, when the small bowel must heal against the abdominal wall to create a seal at the jejunostomy site. The much lower rate of intra-abdominal leak after gastrectomy may be one reason why placement of an FJT does not appear to increase morbidity among patients undergoing gastrectomy.
Several limitations of this study exist. First, using a large retrospective database such as NSQIP contains potential for selection bias because decisions for FJT placement were not determined a priori. Although we attempt to adjust for differences in baseline patient clinical characteristics using propensity score matching, NSQIP lacks certain important patient, tumor, and treatment data (unmeasured variables), which may confound the results. In addition, our data do not contain identifiers for specific hospitals and surgeons. We are therefore not able to evaluate whether greater experience from routine FJT placement may affect perioperative outcomes. Finally, we cannot evaluate concurrent use of total parenteral nutrition, whether FJT placement improved subsequent objective nutritional markers (or body mass index), or if placement of feeding catheter indeed increased adjuvant therapy utilization rates, although these interesting outcomes were not the goals of our study. Despite these limitations, however, we are able to provide by far the largest study to date evaluating FJT placement at the time of gastrectomy for gastric cancer and do not find the combined procedure to result in increased perioperative morbidity or mortality.
5. Conclusions
Our analysis demonstrates that FJT placement at the time of gastrectomy for gastric cancer does not appear to be associated with increased perioperative morbidity or mortality. Reservations regarding the safety profile of FJT should not be the factor limiting their use. FJT placement may allow for increased utilization and completion of adjuvant therapy in the treatment of gastric cancer, but further prospective studies are needed to assess this potential benefit, as well as the effect of FJT placement on other long-term oncologic outcomes. If there are in fact identifiable clinical benefits from alternative feeding access in the adjuvant period, placement of FJT during gastrectomy should be advocated as part of the treatment strategy for gastric cancer.
Footnotes
Authors’ contributions: Z.S., M.M.S., and D.P.N. contributed to the writing of the article. P.J.S. performed the analysis and interpretation. Z.S., D.P.N., J.E.K, B.C.G., P.J.S., D.S.T., and D.G.B. completed the critical revisions.
Disclosure
There are no financial or personal conflicts of interest to declare for the authors.
References
- 1.Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol. 2003;56:1. doi: 10.1016/s0895-4356(02)00534-6. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 3.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 4.Smalley SR, Benedetti JK, Haller DG, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol. 2012;30:2327. doi: 10.1200/JCO.2011.36.7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang Y, Chang H, Yook J, et al. Adjuvant chemotherapy for gastric cancer: a randomised phase 3 trial of mitomycin-C plus either short-term doxifluridine or long-term doxifluridine plus cisplatin after curative D2 gastrectomy (AMC0201) Br J Cancer. 2013;108:1245. doi: 10.1038/bjc.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ajani JA, Bentrem DJ, Besh S, et al. Gastric cancer, version 2. 2013. J Natl Compr Cancer Netw. 2013;11:531. doi: 10.6004/jnccn.2013.0070. [DOI] [PubMed] [Google Scholar]
- 7.Nussbaum D, Zani S, Penne K, et al. Feeding jejunostomy tube placement in patients undergoing pancreaticoduodenectomy: an ongoing dilemma. J Gastrointest Surg. 2014;18:1752. doi: 10.1007/s11605-014-2581-6. [DOI] [PubMed] [Google Scholar]
- 8.Padussis JC, Sabino Z, Blazer DG, et al. Feeding jejunostomy during Whipple is associated with increased morbidity. J Surg Res. 2014;187:361. doi: 10.1016/j.jss.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Patel SH, Kooby DA, Staley CA, 3rd, Maithel SK. An assessment of feeding jejunostomy tube placement at the time of resection for gastric adenocarcinoma. J Surg Oncol. 2013;107:728. doi: 10.1002/jso.23324. [DOI] [PubMed] [Google Scholar]
- 10.Papenfuss WA, Kukar M, Oxenberg J, et al. Morbidity and mortality associated with gastrectomy for gastric Cancer. Ann Surg Oncol. 2014;21:3008. doi: 10.1245/s10434-014-3664-z. [DOI] [PubMed] [Google Scholar]
- 11.Bae JM, Park JW, Yang HK, Kim JP. Nutritional status of gastric cancer patients after total gastrectomy. World J Surg. 1998;22:254. doi: 10.1007/s002689900379. [DOI] [PubMed] [Google Scholar]
- 12.Fenton JR, Bergeron EJ, Coello M, Welsh RJ, Chmielewski GW. Feeding jejunostomy tubes placed during esophagectomy: are they necessary? Ann Thorac Surg. 2011;92:504. doi: 10.1016/j.athoracsur.2011.03.101. [DOI] [PubMed] [Google Scholar]
- 13.Han-Geurts IJ, Verhoef C, Tilanus H. Relaparotomy following complications of feeding jejunostomy in esophageal surgery. Dig Surg. 2004;21:192. doi: 10.1159/000079345. [DOI] [PubMed] [Google Scholar]
- 14.Llaguna OH, Kim H, Deal AM, et al. Utilization and morbidity associated with placement of a feeding jejunostomy at the time of gastroesophageal resection. J Gastrointest Surg. 2011;15:1663. doi: 10.1007/s11605-011-1629-0. [DOI] [PubMed] [Google Scholar]
- 15.Kim HH, Hyung WJ, Cho GS, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report—a phase III multicenter, prospective, randomized trial (KLASS Trial) Ann Surg. 2010;251:417. doi: 10.1097/SLA.0b013e3181cc8f6b. [DOI] [PubMed] [Google Scholar]
- 16.Müller J, Erasmi H, Stelzner M, Zieren U, Pichlmaier H. Surgical therapy of oesophageal carcinoma. Br J Surg. 1990;77:845. doi: 10.1002/bjs.1800770804. [DOI] [PubMed] [Google Scholar]
- 17.Reid-Lombardo KM, Farnell MB, Crippa S, et al. Pancreatic anastomotic leakage after pancreaticoduodenectomy in 1,507 patients: a report from the Pancreatic Anastomotic Leak Study Group. J Gastrointest Surg. 2007;11:1451. doi: 10.1007/s11605-007-0270-4. discussion 1459. [DOI] [PubMed] [Google Scholar]