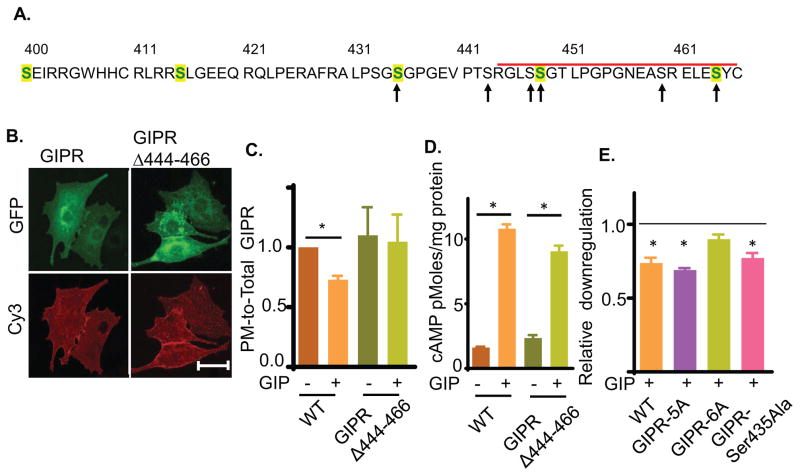
Fig. 5. GIPR intracellular domain is required for GIP induced GIPR sequestration.
(A) Amino acid sequence of GIPR predicted cytoplasmic domain (residues 400 to 466). The C-terminal deletion is marked by the red bar. Serines mutated to alanines are noted by arrows. Serines conserved in human, mouse, rat and bovine GIPR are shown in green. The sequence also contains two threonines as potential phosphorylation sites. However, these threonines are not conserved in mammals.
(B) Images of GIPRΔ444-466-GFP construct in basal adipocytes. Plasma membrane GIPR was labeled by indirect immunofluorescence with anti-HA/Cy3 secondary antibody of fixed cells. Scale bar: 50μm.
(C) Quantification of downregulation of GIPRΔ444-466-GFP constructs upon GIP stimulation. The data are the PM-to-Total of GIP-stimulated normalized to the basal PM-to-Total., P<0.05.
(D) GIP-stimulated cAMP production in adipocytes expressing GIPR or GIPRΔ444-466. The endogenous GIPR was knocked down by siRNA such that cAMP production was downstream of the ectopically expressed GIPRs.
(E) GIP induced downregulation of the GIPR serine mutants. For each construct, the downregulation has been plotted as PM-to-Total normalized to their basal. The unstimulated level (basal) is shown with a dashed line.