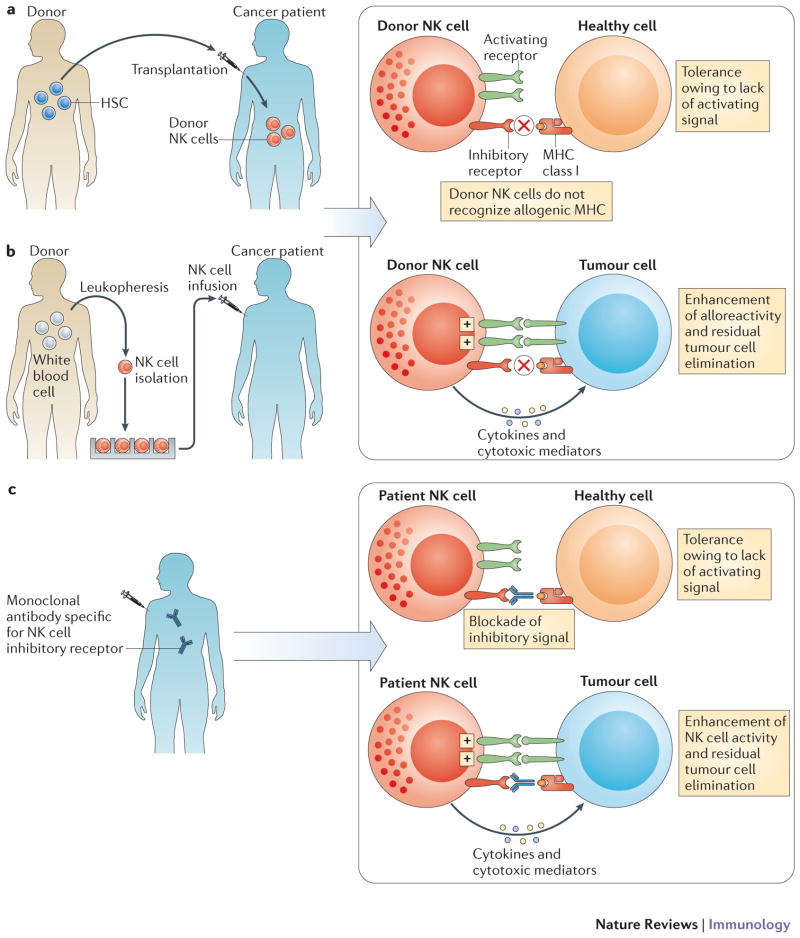
Figure 2.
NK cellular therapy. a | In haplo-identical or MHC-matched HSCT, NK cells of healthy donor origin will develop into the cancer patient. b | Alternatively, NK cells can be isolated from healthy donors, activated and/or expanded in vitro prior to infusion into the cancer patient. In both cases (allo-HSCT and NK cell infusions), the aim is to promote the anti-tumour function of donor NK cells in the cancer patient; indeed a fraction of donor NK cells will be not be inhibited by the MHC class I molecules of the cancer patient as the KIR expressed by these subsets of NK cells will not interact with by the MHC class I molecules of the cancer patient (ELIMINATION). Most normal cells of the patients will not activate donor NK cells as, in contrast to cancer cells of the patient, they lack the cell surface density of ligands required to activate donor NK cells (IGNORANCE). c | Anti-KIR monoclonal antibody therapy. Fully human anti-KIR mAbs can be injected in cancer patient. Anti-KIR mAbs are designed to boost the antitumour activity of NK cells (ELIMINATION) without inducing auto-immunity (IGNORANCE).