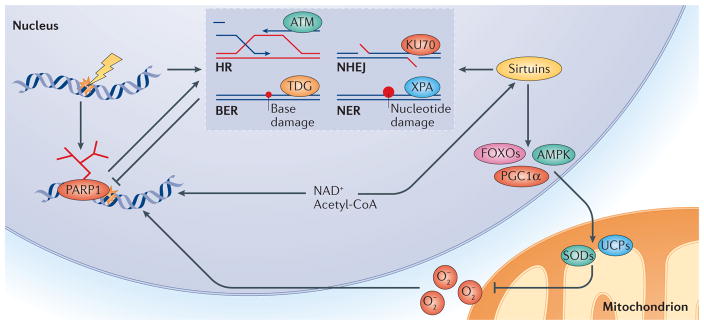
Figure 2. PARP1–NAD+–SIRT1-mediated nuclear DNA damage to mitochondria signalling.
Activation of poly(ADP-ribose) polymerase 1 (PARP1) upon DNA damage facilitates DNA repair but leads to loss of NAD+ and acetyl-CoA, the latter being an important molecule in cellular metabolism. This results in inhibition of sirtuins such as SIRT1, owing to competition for NAD+. Loss of sirtuin activity leads to an increase in mitochondrial reactive oxygen species (O2−), owing to decreased activation of downstream stress response factors such as AMP-activated protein kinase (AMPK), forkhead box O proteins (FOXOs) and peroxisome proliferator-activated receptor-γ co-activator 1α (PGC1α). Collectively, these enzymes regulate a large number of genes that are involved in coping with oxidative stress, such as those encoding uncoupling proteins (UCPs) like UCP2 and superoxide dismutases (SODs). In addition, SIRT1, as well as SIRT6, has been shown to positively regulate several DNA repair pathways, such as homologous recombination (HR), non-homologous end-joining (NHEJ), base excision repair (BER) and nucleotide excision repair (NER). Specifically, SIRT1 has been shown to deacetylate repair factors such as xeroderma pigmentosum group A-complementing protein (XPA, which is involved in NER), KU70 (involved in NHEJ), ataxia telangiectasia mutated (ATM; involved in HR and NHEJ) and thymine DNA glycosylase (TDG; involved in BER). Activation of PARP1 and subsequently less-robust DNA repair, as well as increased oxidative stress, may represent a vicious cycle that could contribute to the ageing process. Thus, PARP1 is pivotal to the initiation of different DNA repair pathways, and it interacts with SIRT1 to execute cellular signalling from the nucleus to mitochondria.