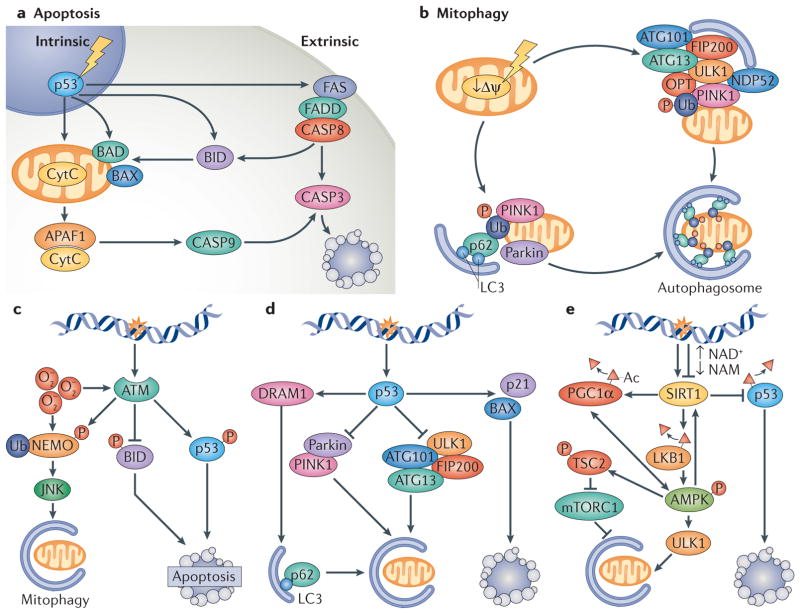
Figure 4. DNA damage signalling in the regulation of mitophagy and apoptosis.
a | Apoptosis can be initiated through intrinsic or extrinsic pathways. The intrinsic pathways can be initiated by genotoxic stress, leading to the activation of p53 and transcriptional upregulation of pro-apoptotic factors such as BAD, BAX and BID. If the stress is sustained or high enough, BAD and BAX accumulation results in the permeabilization of the outer mitochondrial membrane and release of cytochrome C (CytC) that can associate with apoptosis-activating factor 1 (APAF1) and activate caspase 9 (CASP9). CASP9 activation leads to CASP3 activation and initiation of apoptosis. The extrinsic pathway can be initiated through activation of the FAS receptor. This leads to FAS-associated death domain protein (FADD)-mediated activation of CASP8, resulting in CASP3 activation. Notably, there is considerable crosstalk between intrinsic and extrinsic pathways. b | Selective mitophagy is regulated through factors such as PTEN-induced putative kinase 1 (PINK1) and the E3-ubiquitin ligase parkin. Initially, mitochondrial damage causes loss of the mitochondrial membrane potential (Δψ). This leads to the retention of PINK1 at the outer mitochondrial membrane and phosphorylation of outer membrane proteins. Concomitantly, parkin is activated, leading to ubiquitylation of outer membrane proteins. PINK1 phosphorylates ubiquitin that recruits adaptor proteins such as optineurin (OPT), nuclear dot protein 52 (NDP52) and p62, leading to recruitment of the UNC51-like kinase 1 (ULK1) complex (consisting of proteins such as autophagy-related protein 101 (ATG101), ATG13 and FIP200) and to the formation of a double lipid membrane-bound vesicle, the autophagosome. The adaptor proteins (OPT, p62 and NDP52) interact with the autophagosome through light chain 3 (LC3), a small protein that coats the autophagosome. The entire mitochondrion will eventually become engulfed in the autophagosome, which will fuse with a lysosome leading to degradation of the mitochondrion. c | Ataxia telangiectasia mutated (ATM) is activated by breaks in DNA and possibly also by oxidative stress (O2−). At low levels of DNA damage stress, ATM activation leads to phosphorylation, ubiquitylation and activation of the NEMO JNK (NF-κB essential modulator Jun N-terminal kinase) pathway that stimulates mitophagy. ATM also phosphorylates the pro-apoptotic factor BID to inhibit apoptotic signalling. At high levels of stress, ATM phosphorylates and activates p53, which propagates pro-apoptotic signals. d | p53 has been well characterized in the response to genotoxic stimuli, in which it transcriptionally activates pro-apoptotic proteins such as BAX and p21 while simultaneously inhibiting the ULK1-containing autophagy-initiating complex. In addition, p53 activation may lead to decreased expression of parkin and decreased activation of parkin PINK1-mediated mitophagy. At lower levels of stress, p53 can stimulate mitophagy through the activation of DNA damage-regulated autophagy modulator protein 1 (DRAM1), which stimulates p62-mediated mitophagy. e | Sirtuin 1 (SIRT1) regulates both mitophagy and apoptosis. At low levels of DNA damage, nuclear SIRT1 can be activated to facilitate DNA repair. After lethal levels of nuclear DNA damage, SIRT1 is inhibited by the DNA damage response, leading to p53 acetylation and cell death. Loss of SIRT1 activity decreases the stimulation of mitophagy through peroxisome proliferator-activated receptor-γco-activator 1α (PGC1α)- and AMP-activated kinase (AMPK)-dependent pathways. SIRT1 regulates mitochondrial biogenesis and mitophagy through deacetylation of PGC1α, and it also interacts with AMPK to regulate mitophagy through mutual activation: AMPK activates SIRT1 by increasing the ratio of NAD+/NADH, and it is activated by SIRT1 through deacetylation of liver kinase B1 (LKB1), an AMPK activator. Furthermore, AMPK phosphorylates and activates the mTORC1 inhibitor protein tuberous sclerosis complex 2 (TSC2), thereby further activating autophagy. AMPK also positively regulates PGC1α activity, which stimulates mitochondrial biogenesis and activates forkhead box protein O 3A, leading to activation of stress response and pro-survival pathways. NAM, nicotinamide.