Abstract
The breadth of unprecedented enzymatic reactions performed during the formation of microbial natural products has continued to expand as new biosynthetic gene clusters are unearthed by genome mining. Enzymes that use aminoacyl-tRNA (aa-tRNA) outside of the translation machinery have been known for decades, but accounts of their use in natural product biosynthesis are just beginning to accumulate. This review will highlight the recent discoveries and advances in our mechanistic understanding of aa-tRNA-dependent enzymes that play key roles in the biosynthesis of a growing number of microbial natural products.
Introduction
Investigations of enzyme reaction mechanisms involved in natural product biosynthesis continue to reveal a fascinating toolbox for the production of structural diversity [1]. One example is Nature’s ability to find new uses for aminoacyl-transfer ribonucleic acid (aa-tRNA) as a macromolecular activated co-substrate that can donate an amino acid for amide or ester bond formation. Considering that aa-tRNAs have profound importance for the cell as the link between mRNA and peptide formation at the ribosome, it is intriguing that aa-tRNA is also accepted as a substrate for enzymes involved in cell wall biosynthesis, antibiotic resistance, porphyrin biosynthesis, protein degradation pathways, and antibiotic biosynthesis [2–6]. These topics are covered in a number of comprehensive recent reviews [7–9]; this review will focus on the use of aa-tRNA in natural product biosynthesis. The realization that aa-tRNA is used in these processes started in 2008, with this review emphasizing studies published in the period 2014–2016.
Cyclodipeptide Synthases
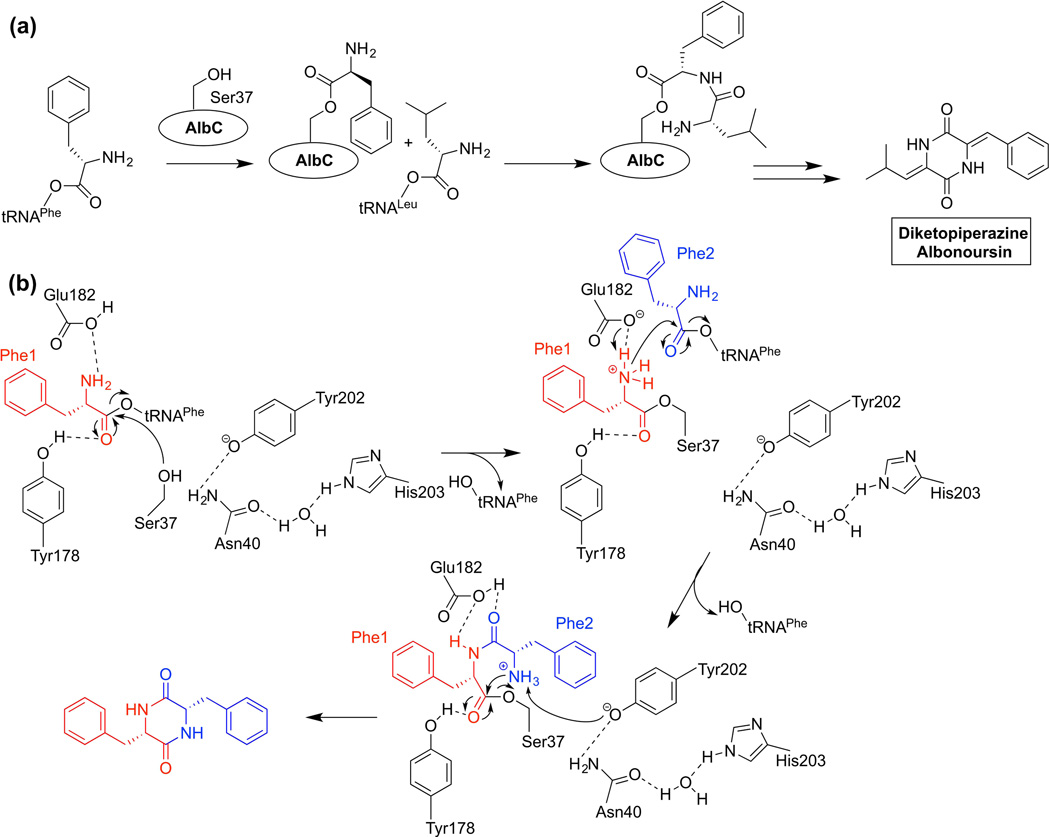
An analysis of gene clusters involved in the production of diketopiperazines led to the discovery of a putative cluster that did not contain the canonical nonribosomal peptide synthetase (NRPS) responsible for formation of the cyclodipeptide scaffold in all CDP biosynthetic pathways that were known at the time [10]. Follow-up studies revealed a family of much shorter proteins that utilize amino acids pre-activated by aminoacyl-tRNA synthetases (aaRSs) (Figure 1) [11–13]. These Cyclodipeptide Synthases (CDPSs) have structural homology with TyrRS and TrpRS proteins from which they appear to have evolved [14–17]. A total of 54 CDPSs have been assayed for activity to date, with producing organisms spread across six bacterial phyla and one eukaryotic species [11,14,15,18–22]. The cyclodipeptide products formed by these proteins are comprised of 17 different proteinogenic amino acids [18]. The mechanism of CDPSs involves the sequential interaction with two aa-tRNAs at different binding sites and the initial formation of a covalent enzyme-amino acid intermediate at a conserved serine residue (Figure 1a) [14–16,23].
Figure 1.
(a) General cyclodipeptide synthase reaction scheme using AlbC from Streptomyces noursei as an example. The reaction proceeds via a dipeptidyl-enzyme intermediate covalently linked to the indicated conserved serine. (b) Proposed mechanism and active site residues involved in the AlbC interaction with two Phe-tRNAPhe substrates. Figure adapted from [24].
A recent co-crystal structure of the Streptomyces noursei CDPS AlbC with a dipeptidyl intermediate analog led to the identification of a diphenylalanyl-enzyme intermediate rather than a dipeptidyl-tRNA intermediate after interaction with the second Phe-tRNAPhe [24]. A conserved tyrosine (Tyr202) has been proposed to participate in activating the amine of substrate Phe2 for nucleophilic attack on Phe1 during the cyclization step (Figure 1b). Reaction of an AlbC-Y202F variant with Phe-tRNAPhe resulted in successful trapping of a diphenylalanyl-enzyme intermediate with the dipeptide moiety attached to Ser37. The positioning of the substrate analog also allowed for the evaluation of other key residues lining the active site pocket, such as Asn40 and His203 (Figure 1b) [24].
The combined efforts in CDPS structural biology and biochemistry have led to attempts to predict substrate specificity of yet-to-be characterized CDPSs [18]. A recent analysis classified characterized and putative CDPSs into two main families based on the identity of conserved active site residues [18]. Within a given family, the use of a certain amino acid substrate at either the first or second binding site was correlated with a predictable set of residues lining the binding pocket [18,24]. Ten putative CDPSs were then tested and the predicted cyclodipeptide structure was the major product formed in all cases. The continued influx of new genetic information will likely increase the number of CDPS homologs and strengthen this predictive model.
Structural Homologs to FemX and MprF
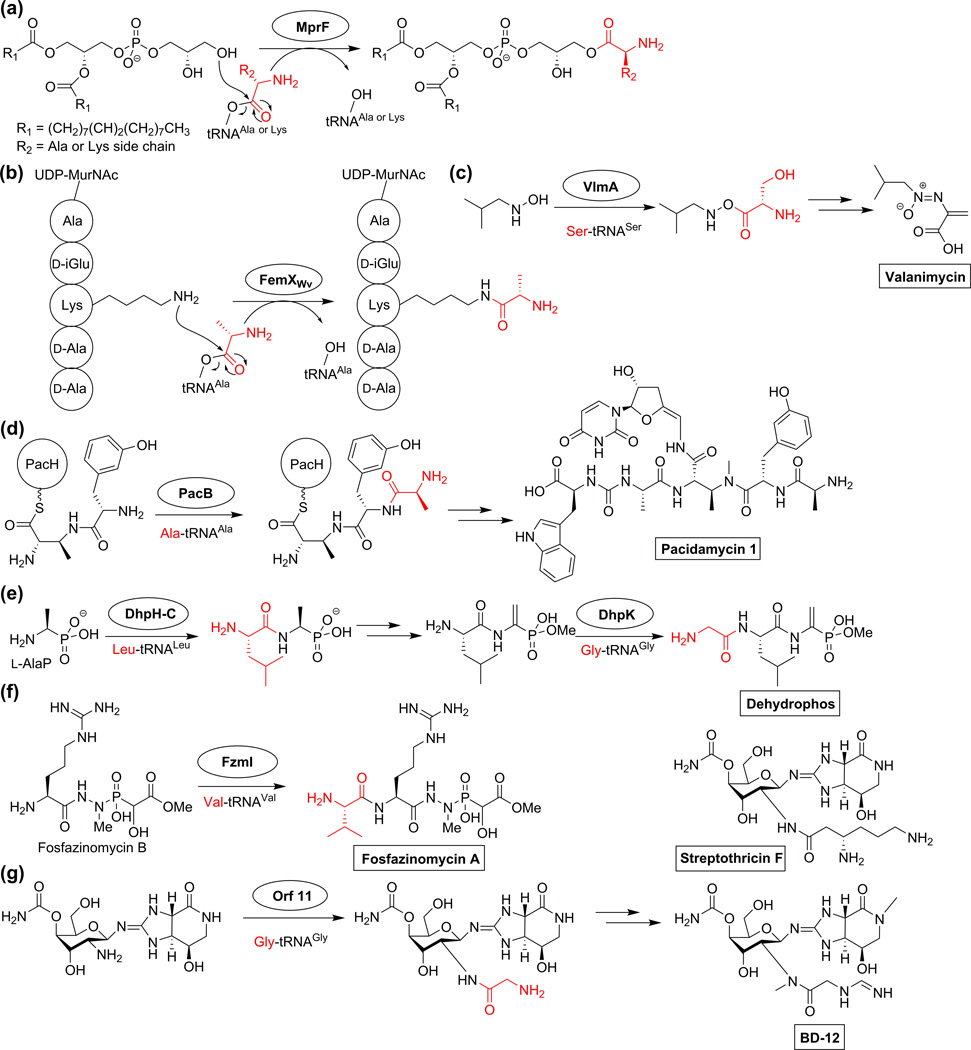
A number of other natural product biosynthetic enzymes that utilize aa-tRNA are structural homologs of MprF (multiple peptide resistance factor) and FemX (factors essential for methicillin resistance) that share the GCN5-related N-acetyltransferase (GNAT) fold. Although no general sequence conservation or aa-tRNA binding region has been identified, recent co-crystal structures with substrate analogs point to common residues such as phenylalanine and lysine involved in electrostatic interactions with the aminoacyl-acceptor stem region [25,26]. The MprF proteins catalyze the transfer of alanine and lysine from aa-tRNA to phosphatidylglycerol in the cell membrane to neutralize the negative charge of the phospholipid bilayer and decrease the permeability of positively charged antimicrobial peptides (Figure 2a) [27]. The cell wall biosynthesis enzyme FemX from Weissella viridescens catalyzes the formation of branched peptide chains by conjugating the ε-amino group of lysine in UDP-MurNAc pentapeptide with l-Ala from Ala-tRNAAla (Figure 2b) [28]. Other members of the FemABX family catalyze similar reactions but use different aa-tRNA substrates [9]. The following subsections will highlight the MprF/FemX homologs that are involved in biosynthesis of a diverse set of natural products.
Figure 2.
Proposed mechanism of aminoacyl transfer in the activity of FemXWv and MprF, along with the diverse reactions performed by homologous biosynthetic enzymes involved in natural product biosynthesis. Streptothricin F (which is produced by NRPS machinery) is shown for comparison with the derivative BD-12. The atoms of the final natural product that originated from aminoacyl-tRNA-dependent activity are highlighted in red.
VlmA in Valanimycin Biosynthesis
The use of aa-tRNA in natural product biosynthesis was first noted for the production of valanimycin by Streptomyces viridifaciens MG456-hF10 (Figure 2c) [5]. The valanimycin gene cluster surprisingly contains an extra copy of SerRS (VlmL) in addition to the SerRS used for protein synthesis [29]. This discovery prompted the search for an enzyme in the valanimycin biosynthetic pathway that would accept Ser-tRNASer as a substrate. VlmA was proposed as the candidate enzyme to accept aa-tRNA due to its weak homology with MprF. Based on previous knowledge of the valanimycin pathway, VlmA activity was reconstituted in vitro and shown to attach l-serine to isobutylhydroxylamine (Figure 2c).
PacB in Pacidamycin Biosynthesis
Pacidamycins are nucleoside antibiotics with a uridyl moiety and a tetra- or pentapeptide chain containing a diaminobutyric acid link (Figure 2d) [30]. The gene cluster from Streptomyces coeruleorubidus NRRL 18370 revealed an NRPS scaffold that is responsible for production of a uridyl tetrapeptide, but an extra enzyme is required to add the fifth amino acid [30]. PacB shares 11% identity with FemX and uses Ala–tRNAAla for this fifth peptide bond formation. Although PacB uses an alternative mechanism to the NRPS machinery, it does still require a substrate that is tethered to the pantetheinyl arm of the peptidyl carrier protein PacH.
DhpH-C and DhpK in Dehydrophos Biosynthesis
The tripeptide phosphonate natural product dehydrophos produced by Streptomyces luridus requires the use of two aa-tRNA-dependent enzymes for its biosynthesis [31]. The C-terminal domain of DhpH (DhpH-C) and DhpK catalyze amide bond formation using Leu-tRNALeu and Gly-tRNAGly, respectively (Figure 2e). The enzymes share only 16% sequence identity, yet both are structural homologs of FemX family members, which was key to their functional assignment. DhpH-C was shown to act on the phosphonate derivative of alanine (l-AlaP) to make a dipeptide, with DhpK adding glycine to make the final tripeptide [31].
FzmI in Fosfazinomycin Biosynthesis
Another example of a compound in which Nature has utilized a variety of different strategies to assemble a natural product is fosfazinomycin. Its gene cluster contains an ATP-GRASP enzyme, a FemX homolog, and a glutamine synthetase that are thought to form the three amide/hydrazide linkages [32]. Only the activity of the FemX enzyme is currently established and it adds the N-terminal Val in a Val-tRNAVal dependent process (Figure 2f) [33].
Orf 11 in Streptothricin Biosynthesis
Like CDPS biosynthesis, the biosynthesis of amide bond formation in streptothricin and its derivatives (Figure 2g) can occur by two different methods. The biosynthetic pathway of streptothricins include NRPS modules to make a linkage between an amino sugar and β-lysine [34]. However, a gene cluster found in Streptomyces luteocolor NBRC13826 that makes the analog BD-12 does not contain NRPS machinery [35]. Instead, structural homology searches identified Orf 11 as a homolog of FemX that is responsible for the addition of glycine to the BD-12 structure.
Glutamyl-tRNA-Dependent Enzymes in RiPP Biosynthesis
Lanthipeptides
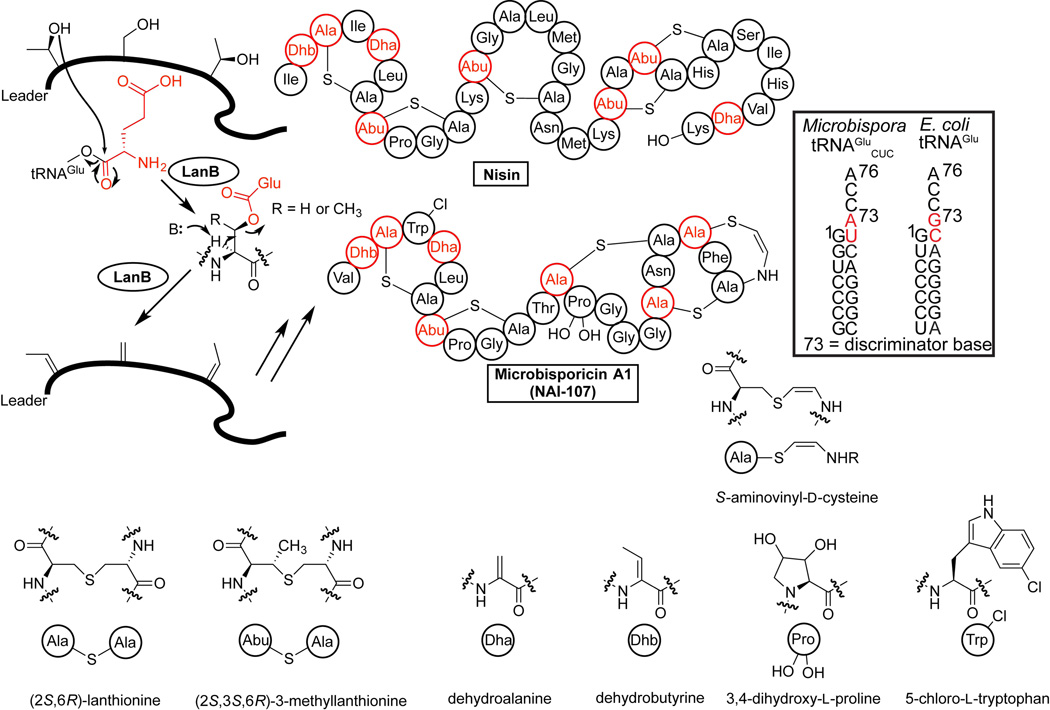
Surprisingly, aa-tRNA was discovered to be a substrate for enzymes involved in the post-translational modification of several RiPPs (ribosomally synthesized and post-translationally modified peptides) [36]. Unlike the processes described thus far, these enzymes only transiently attach amino acids to their substrates in an aa-tRNA dependent fashion, but the amino acids do not end up in the final compounds. The first discovery of these enzymes was during investigations of the lantibiotic nisin, which contains dehydroalanine and dehydrobutyrine residues resulting from the NisB-catalyzed dehydration of serines and threonines, respectively, in the peptide NisA (Figure 3). NisB is a family member of the lanthionine biosynthesis enzymes (LanB), which show no overall homology to other protein domains and hence unlike the FemX-homologs were not anticipated to use aa-tRNA [36]. In order to activate the side chains of Ser and Thr for the dehydration reaction, the residues are glutamylated by a transesterification reaction with Glu-tRNAGlu [36,37]. The N-terminal domain of NisB is responsible for this glutamylation, while the C-terminal domain is involved in subsequent Glu elimination to form the dehydrated residue. NisB can accept Glu-tRNAGlu from both E. coli and the native producer Lactococcus lactis [36].
Figure 3.
Proposed mechanism of glutamylation and elimination by LanB dehydratases during biosynthesis of the lantibiotics nisin and microbisporicin. Ser and Thr residues in a precursor peptide are glutamylated in a Glu-tRNAGlu-dependent reaction. The glutamate is then eliminated to generate dehydroalanine and dehydrobutyrine redidues. The inset next to microbisporicin A1 shows the sequence of the tRNA acceptor stems for Microbispora and E. coli tRNAGlu with bases predicted to be important for recognition highlighted in red. The glutamate elimination reaction is shown as a concerted process but could involve an enolate intermediate. After dehydration, other enzymes carry out additional post-translational modifications (PTMs) that result in the structures shown at the bottom. The structures for the shorthand notations used in the nisin and microbisporicin drawings are shown.
Attempts to produce lantibiotics in heterologous hosts such as E. coli has provided insights into tRNA recognition. Unlike nisin, the dehydratase MibB involved in the biosynthesis of microbisporicin A1 does not accept Glu-tRNAGlu from E. coli [38]. Microbisporicin is produced by Microbispora sp. 107891 and is in pre-clinical trials for use against multi-drug-resistant Gram-positive bacterial infections [39]. When MibB was incubated with the Microbispora isoacceptor tRNAGluCUC and its corresponding GluRS, all seven expected dehydrations of the substrate peptide MibA were observed. Comparison of the Microbispora tRNAGluCUC substrate with E. coli tRNAGlu suggested that the acceptor stem sequence might be important for recognition by MibB (Figure 3). Consistent with this hypothesis, previous work on FemX, MprF, CDPS, and a NisB-tRNA docking model had suggested that the aminoacyl moiety and the acceptor stem are the main areas of contact between these enzymes and aa-tRNA [23,36,40,41]. Mutagenesis experiments and subsequent activity assays with MibB supports the recognition of the discriminator base and the preceding base of the acceptor stem [38]. Hence in at least two different phyla (Firmicutes for nisin and Actinobacteria for microbisporicin), Glu-tRNAGlu is required for dehydration. The reason why activation with Glu-tRNAGlu evolved for this relatively easy reaction is at present not understood.
Thiopeptides and Goadsporin
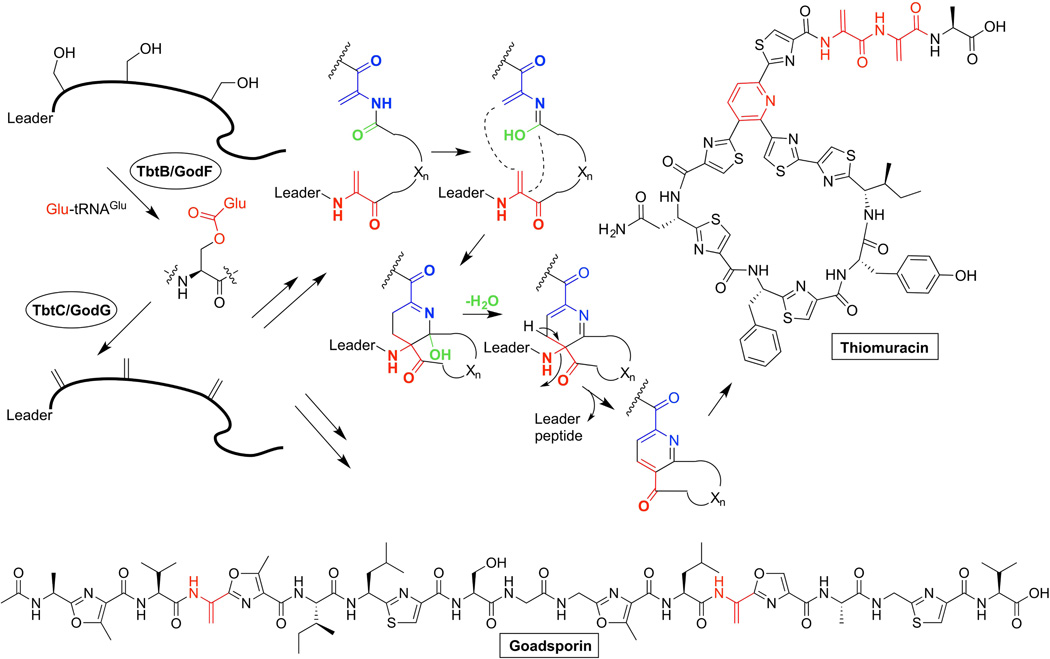
The thiopeptide class of RiPP natural products also requires the use of aa-tRNA for biosynthesis of dehydroalanine residues [42]. During biosynthesis, two dehydroalanine residues form a nitrogen-containing six-membered heterocycle via a formal [4+2] cycloaddition [42–45]. The biosynthesis of the thiopeptide thiomuracin from Thermobispora bispora DSM 43833 was recently reconstituted in vitro (Figure 4) [42]. Its gene cluster encodes a split LanB-type protein consisting of TbtB and TbtC, which correspond to the glutamylation and elimination domains of NisB, respectively. As seen with MibB activity, TbtB did not accept E. coli Glu-tRNAGlu, but all four dehydrations of the thiomuracin precursor were accomplished when the T. bispora isoacceptor tRNAGluCUC was used for aa-tRNA generation.
Figure 4.
Proposed activity of split-LanB dehydratases. The split-LanBs act on their substrates as part of a hybrid system with thiazole/oxazole-forming machinery. The proposed mechanism for the heterocycle formation from two dehydroalanine residues is shown.
Goadsporin is another RiPP that contains dehydroalanines formed via glutamylation (Figure 4) [46]. Goadsporin is produced by Streptomyces sp. TP-A0584 and consists of a linear peptide with azole and dehydroalanine modifications. Like thiopeptides, the gene cluster encodes for split LanB-type enzymes GodF and GodG. To investigate the function of these enzymes, a godF and godG double mutant and a godG single mutant were constructed in a recombinant Streptomyces lividans strain that contains the machinery to produce goadsporin [46]. Goadsporin contains two dehydroalanines and the natural product isolated from the double mutant strain contained two preserved serine residues. The godG single mutant strain produced a peptide that was mono-glutamylated at Ser4. Finally, the chemical structure of the glutamylated serine was elucidated using NMR spectroscopy. Thus, the use of Glu-tRNAGlu for dehydration of Ser and Thr in RiPPs appears widespread.
Conclusions and Outlook
The past eight years have shown that aa-tRNA-dependent enzymes operate in the biosynthesis of a diverse set of natural products. The gene clusters that harbor these enzymes sometimes contain dedicated aaRSs, aa-tRNA-dependent domains fused to other gene products, multiple aa-tRNA-dependent proteins, and/or a mixture of amide-bond-forming enzyme families, illustrating the wide variety of ways in which Nature has recruited aa-tRNA machinery to natural product biosynthesis. This diversity is likely to continue to expand as new data emerges. For example, a gene cluster for the nucleoside antibiotic ascamycin was recently found to contain two genes with homology to aaRSs and a gene encoding a protein that does not have homology with any of the enzymes discussed in this review. This latter protein may depend on aa-tRNA for formation of a final amide bond [47]. Discovery of the requirement of aa-tRNA in biosynthetic pathways is not straightforward when such clues are missing from the gene clusters. However, now that the use of aa-tRNA-dependent enzymes in biosynthetic pathways has been increasingly recognized, it is likely that more strategies that rely on aa-tRNA will be discovered.
Highlights.
Aminoacyl-tRNA (aa-tRNA) is involved in the production of diverse natural products.
Cyclodipeptide synthases interact with two aa-tRNAs at separate binding sites.
Many natural product gene clusters encode FemX/MprF homologs that require aa-tRNA.
Different RiPP classes require aa-tRNA for Ser/Thr dehydration.
Acknowledgments
The authors would like to thank Dr. Manuel Ortega and Kenton Hetrick (UIUC) for critically reviewing the manuscript. Work from the author’s laboratory was supported by the National Institutes of Health (GM P01 077596) and (GM R01 058822).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Corre C, Challis GL. New natural product biosynthetic chemistry discovered by genome mining. Nat Prod Rep. 2009;26:977–986. doi: 10.1039/b713024b. [DOI] [PubMed] [Google Scholar]
- 2.Plapp R, Strominger JL. Biosynthesis of the peptidoglycan of bacterial cell walls. XVII. Biosynthesis of peptidoglycan and of interpeptide bridges in Lactobacillus viridescens. J Biol Chem. 1970;245:3667–3674. [PubMed] [Google Scholar]
- 3.Schon A, Krupp G, Gough S, Berry-Lowe S, Kannangara CG, Soll D. The RNA required in the first step of chlorophyll biosynthesis is a chloroplast glutamate tRNA. Nature. 1986;322:281–284. doi: 10.1038/322281a0. [DOI] [PubMed] [Google Scholar]
- 4.Leibowitz MJ, Soffer RL. Enzymatic modification of proteins. 3. Purification and properties of a leucyl, phenylalanyl transfer ribonucleic acid protein transferase from Escherichia coli. J Biol Chem. 1970;245:2066–2073. [PubMed] [Google Scholar]
- 5.Garg RP, Qian XL, Alemany LB, Moran S, Parry RJ. Investigations of valanimycin biosynthesis: elucidation of the role of seryl-tRNA. Proc Natl Acad Sci U S A. 2008;105:6543–6547. doi: 10.1073/pnas.0708957105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lennarz WJ, Nesbitt JA, 3rd, Reiss J. The participation of sRNA in the enzymatic synthesis of O-L-lysyl phosphatidylgylcerol in Staphylococcus aureus. Proc Natl Acad Sci U S A. 1966;55:934–941. doi: 10.1073/pnas.55.4.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raina M, Ibba M. tRNAs as regulators of biological processes. Front Genet. 2014;5:171. doi: 10.3389/fgene.2014.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shepherd J, Ibba M. Direction of aminoacylated transfer RNAs into antibiotic synthesis and peptidoglycan-mediated antibiotic resistance. FEBS Lett. 2013;587:2895–2904. doi: 10.1016/j.febslet.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dare K, Ibba M. Roles of tRNA in cell wall biosynthesis. Wiley Interdiscip Rev RNA. 2012;3:247–264. doi: 10.1002/wrna.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lautru S, Gondry M, Genet R, Pernodet JL. The albonoursin gene cluster of S. noursei: biosynthesis of diketopiperazine metabolites independent of nonribosomal peptide synthetases. Chem Biol. 2002;9:1355–1364. doi: 10.1016/s1074-5521(02)00285-5. [DOI] [PubMed] [Google Scholar]
- 11.Gondry M, Sauguet L, Belin P, Thai R, Amouroux R, Tellier C, Tuphile K, Jacquet M, Braud S, Courcon M, et al. Cyclodipeptide synthases are a family of tRNA-dependent peptide bond-forming enzymes. Nat Chem Biol. 2009;5:414–420. doi: 10.1038/nchembio.175. [DOI] [PubMed] [Google Scholar]
- 12.Belin P, Moutiez M, Lautru S, Seguin J, Pernodet JL, Gondry M. The nonribosomal synthesis of diketopiperazines in tRNA-dependent cyclodipeptide synthase pathways. Nat Prod Rep. 2012;29:961–979. doi: 10.1039/c2np20010d. [DOI] [PubMed] [Google Scholar]
- 13.Giessen TW, Marahiel MA. The tRNA-dependent biosynthesis of modified cyclic dipeptides. Int J Mol Sci. 2014;15:14610–14631. doi: 10.3390/ijms150814610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vetting MW, Hegde SS, Blanchard JS. The structure and mechanism of the Mycobacterium tuberculosis cyclodityrosine synthetase. Nat Chem Biol. 2010;6:797–799. doi: 10.1038/nchembio.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonnefond L, Arai T, Sakaguchi Y, Suzuki T, Ishitani R, Nureki O. Structural basis for nonribosomal peptide synthesis by an aminoacyl-tRNA synthetase paralog. Proc Natl Acad Sci U S A. 2011;108:3912–3917. doi: 10.1073/pnas.1019480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sauguet L, Moutiez M, Li Y, Belin P, Seguin J, Le Du MH, Thai R, Masson C, Fonvielle M, Pernodet JL, et al. Cyclodipeptide synthases, a family of class-I aminoacyl-tRNA synthetase-like enzymes involved in non-ribosomal peptide synthesis. Nucleic Acids Res. 2011;39:4475–4489. doi: 10.1093/nar/gkr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aravind L, de Souza RF, Iyer LM. Predicted class-I aminoacyl tRNA synthetase-like proteins in non-ribosomal peptide synthesis. Biol Direct. 2010;5:48. doi: 10.1186/1745-6150-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jacques IB, Moutiez M, Witwinowski J, Darbon E, Martel C, Seguin J, Favry E, Thai R, Lecoq A, Dubois S, et al. Analysis of 51 cyclodipeptide synthases reveals the basis for substrate specificity. Nat Chem Biol. 2015;11:721–727. doi: 10.1038/nchembio.1868. This study presents a large-scale analysis of cyclodipeptide synthases and predicts their substrate specificity based on shared binding pocket residues..
- 19.Giessen TW, von Tesmar AM, Marahiel MA. A tRNA-dependent two-enzyme pathway for the generation of singly and doubly methylated ditryptophan 2,5-diketopiperazines. Biochemistry. 2013;52:4274–4283. doi: 10.1021/bi4004827. [DOI] [PubMed] [Google Scholar]
- 20.Giessen TW, von Tesmar AM, Marahiel MA. Insights into the generation of structural diversity in a tRNA-dependent pathway for highly modified bioactive cyclic dipeptides. Chem Biol. 2013;20:828–838. doi: 10.1016/j.chembiol.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Seguin J, Moutiez M, Li Y, Belin P, Lecoq A, Fonvielle M, Charbonnier JB, Pernodet JL, Gondry M. Nonribosomal peptide synthesis in animals: the cyclodipeptide synthase of Nematostella. Chem Biol. 2011;18:1362–1368. doi: 10.1016/j.chembiol.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 22.James ED, Knuckley B, Alqahtani N, Porwal S, Ban J, Karty JA, Viswanathan R, Lane AL. Two distinct cyclodipeptide synthases from a marine Actinomycete catalyze biosynthesis of the same diketopiperazine natural product. ACS Synth Biol. 2015 doi: 10.1021/acssynbio.5b00120. [DOI] [PubMed] [Google Scholar]
- 23. Moutiez M, Seguin J, Fonvielle M, Belin P, Jacques IB, Favry E, Arthur M, Gondry M. Specificity determinants for the two tRNA substrates of the cyclodipeptide synthase AlbC from Streptomyces noursei. Nucleic Acids Res. 2014;42:7247–7258. doi: 10.1093/nar/gku348. This study determined that two separate binding sites are used to interact with each aminoacyl-tRNA substrate.
- 24. Moutiez M, Schmitt E, Seguin J, Thai R, Favry E, Belin P, Mechulam Y, Gondry M. Unravelling the mechanism of non-ribosomal peptide synthesis by cyclodipeptide synthases. Nat Commun. 2014;5:5141. doi: 10.1038/ncomms6141. In this study, the authors provide evidence of a dipeptidyl-enzyme intermediate in cyclodipeptide synthase activity. This distinguishes between the mechanistic hypotheses of a dipeptidyl-enzyme or dipeptidyl-tRNA intermediate.
- 25.Fonvielle M, Li de La Sierra-Gallay I, El-Sagheer AH, Lecerf M, Patin D, Mellal D, Mayer C, Blanot D, Gale N, Brown T, et al. The structure of FemX(Wv) in complex with a peptidyl-RNA conjugate: mechanism of aminoacyl transfer from Ala-tRNA(Ala) to peptidoglycan precursors. Angew Chem Int Ed Engl. 2013;52:7278–7281. doi: 10.1002/anie.201301411. [DOI] [PubMed] [Google Scholar]
- 26.Hebecker S, Krausze J, Hasenkampf T, Schneider J, Groenewold M, Reichelt J, Jahn D, Heinz DW, Moser J. Structures of two bacterial resistance factors mediating tRNA-dependent aminoacylation of phosphatidylglycerol with lysine or alanine. Proc Natl Acad Sci U S A. 2015;112:10691–10696. doi: 10.1073/pnas.1511167112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roy H, Ibba M. RNA-dependent lipid remodeling by bacterial multiple peptide resistance factors. Proc Natl Acad Sci U S A. 2008;105:4667–4672. doi: 10.1073/pnas.0800006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hegde SS, Shrader TE. FemABX family members are novel nonribosomal peptidyltransferases and important pathogen-specific drug targets. J Biol Chem. 2001;276:6998–7003. doi: 10.1074/jbc.M008591200. [DOI] [PubMed] [Google Scholar]
- 29.Garg RP, Gonzalez JM, Parry RJ. Biochemical characterization of VlmL, a seryl-tRNA synthetase encoded by the valanimycin biosynthetic gene cluster. J Biol Chem. 2006;281:26785–26791. doi: 10.1074/jbc.M603675200. [DOI] [PubMed] [Google Scholar]
- 30.Zhang W, Ntai I, Kelleher NL, Walsh CT. tRNA-dependent peptide bond formation by the transferase PacB in biosynthesis of the pacidamycin group of pentapeptidyl nucleoside antibiotics. Proc Natl Acad Sci U S A. 2011;108:12249–12253. doi: 10.1073/pnas.1109539108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bougioukou DJ, Mukherjee S, van der Donk WA. Revisiting the biosynthesis of dehydrophos reveals a tRNA-dependent pathway. Proc Natl Acad Sci U S A. 2013;110:10952–10957. doi: 10.1073/pnas.1303568110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao J, Ju KS, Yu X, Velasquez JE, Mukherjee S, Lee J, Zhao C, Evans BS, Doroghazi JR, Metcalf WW, et al. Use of a phosphonate methyltransferase in the identification of the fosfazinomycin biosynthetic gene cluster. Angew Chem Int Ed Engl. 2014;53:1334–1337. doi: 10.1002/anie.201308363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang Z, Wang K-KA, van der Donk WA. New insights into the biosynthesis of fosfazinomycin. Chemical Science. 2016 doi: 10.1039/c6sc01389a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maruyama C, Toyoda J, Kato Y, Izumikawa M, Takagi M, Shin-ya K, Katano H, Utagawa T, Hamano Y. A stand-alone adenylation domain forms amide bonds in streptothricin biosynthesis. Nat Chem Biol. 2012;8:791–797. doi: 10.1038/nchembio.1040. [DOI] [PubMed] [Google Scholar]
- 35. Maruyama C, Niikura H, Izumikawa M, Hashimoto J, Shin-Ya K, Komatsu M, Ikeda H, Kuroda M, Sekizuka T, Ishikawa J, et al. tRNA-dependent aminoacylation of an amino-sugar intermediate in the biosynthesis of a streptothricin-related antibiotic. Appl Environ Microbiol. 2016;82:3640–3648. doi: 10.1128/AEM.00725-16. Thee authors show that a streptothricin-analog is biosynthesized in a different manner that streptothricin in that aminoacyltRNA is used for amide bond formation rather than the NRPS mechanism used in other streptothricins.
- 36. Ortega MA, Hao Y, Zhang Q, Walker MC, van der Donk WA, Nair SK. Structure and mechanism of the tRNA-dependent lantibiotic dehydratase NisB. Nature. 2015;517:509–512. doi: 10.1038/nature13888. This report reveals the unexpected finding that aminoacyl-tRNA is involved in activating Ser/Thr for dehydration in ribosomally synthesized and post-translationally modified peptide production.
- 37.Garg N, Salazar-Ocampo LM, van der Donk WA. In vitro activity of the nisin dehydratase NisB. Proc Natl Acad Sci U S A. 2013;110:7258–7263. doi: 10.1073/pnas.1222488110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ortega MA, Hao Y, Walker MC, Donadio S, Sosio M, Nair SK, van der Donk WA. Structure and tRNA specificity of MibB, a lantibiotic dehydratase from Actinobacteria involved in NAI-107 biosynthesis. Cell Chem Biol. 2016;23:370–380. doi: 10.1016/j.chembiol.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jabes D, Brunati C, Candiani G, Riva S, Romano G, Donadio S. Efficacy of the new lantibiotic NAI-107 in experimental infections induced by multidrug-resistant Gram-positive pathogens. Antimicrob Agents Chemother. 2011;55:1671–1676. doi: 10.1128/AAC.01288-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villet R, Fonvielle M, Busca P, Chemama M, Maillard AP, Hugonnet JE, Dubost L, Marie A, Josseaume N, Mesnage S, et al. Idiosyncratic features in tRNAs participating in bacterial cell wall synthesis. Nucleic Acids Res. 2007;35:6870–6883. doi: 10.1093/nar/gkm778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hebecker S, Arendt W, Heinemann IU, Tiefenau JH, Nimtz M, Rohde M, Soll D, Moser J. Alanyl-phosphatidylglycerol synthase: mechanism of substrate recognition during tRNA-dependent lipid modification in Pseudomonas aeruginosa. Mol Microbiol. 2011;80:935–950. doi: 10.1111/j.1365-2958.2011.07621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hudson GA, Zhang Z, Tietz JI, Mitchell DA, van der Donk WA. In vitro biosynthesis of the core scaffold of the thiopeptide thiomuracin. J Am Chem Soc. 2015;137:16012–16015. doi: 10.1021/jacs.5b10194. This study showed that Glu-tRNA is also used for dehydration of Ser/Thr residues during thiopeptide biosynthesis.
- 43.Bycroft BW, Gowland MS. The structures of the highly modified peptide antibiotics micrococcin P1 and P2. J Chem Soc, Chem Commun. 1978:256–258. [Google Scholar]
- 44.Bowers AA, Walsh CT, Acker MG. Genetic interception and structural characterization of thiopeptide cyclization precursors from Bacillus cereus. J Am Chem Soc. 2010;132:12182–12184. doi: 10.1021/ja104524q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wever WJ, Bogart JW, Baccile JA, Chan AN, Schroeder FC, Bowers AA. Chemoenzymatic synthesis of thiazolyl peptide natural products featuring an enzyme-catalyzed formal [4 + 2] cycloaddition. J Am Chem Soc. 2015;137:3494–3497. doi: 10.1021/jacs.5b00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ozaki T, Kurokawa Y, Hayashi S, Oku N, Asamizu S, Igarashi Y, Onaka H. Insights into the biosynthesis of dehydroalanines in goadsporin. Chembiochem. 2016;17:218–223. doi: 10.1002/cbic.201500541. This study reports the direct detection of glutamylated intermediates, illustrating that the use of Glu-tRNA is not restricted to lanthipeptide dehydration.
- 47.Zhao C, Qi J, Tao W, He L, Xu W, Chan J, Deng Z. Characterization of biosynthetic genes of ascamycin/dealanylascamycin featuring a 5'-O-sulfonamide moiety in Streptomyces sp. JCM9888. PLoS One. 2014;9:e114722. doi: 10.1371/journal.pone.0114722. [DOI] [PMC free article] [PubMed] [Google Scholar]