Extended Data Figure 2. Stereochemical structure-activity relationship.
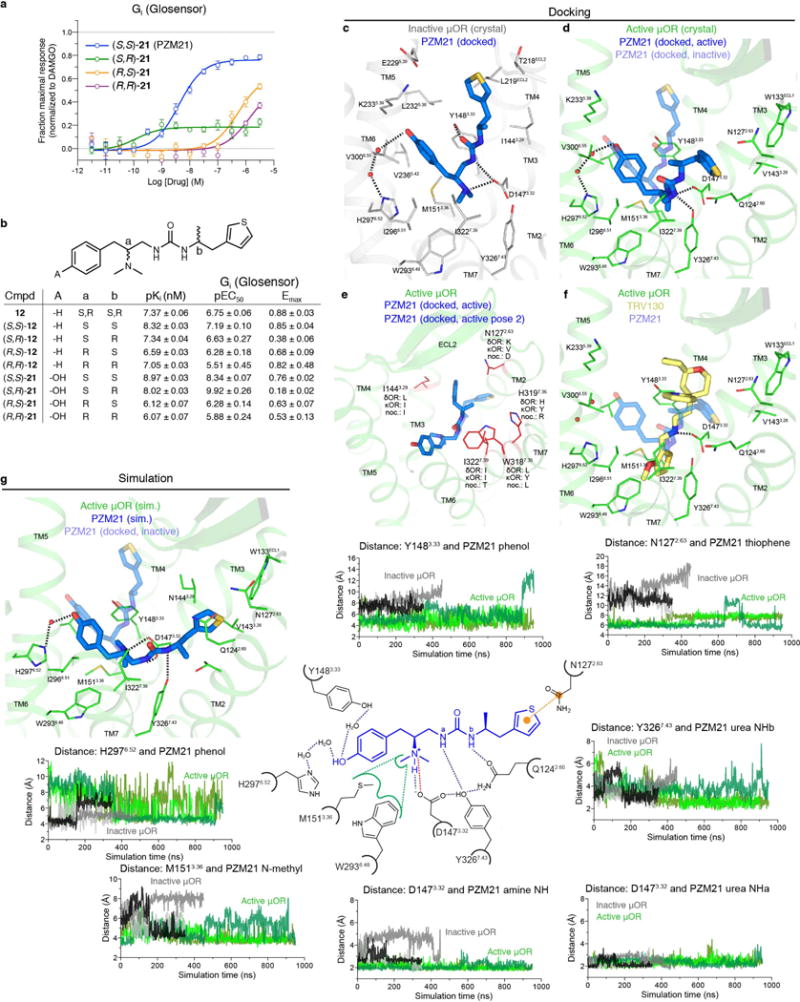
a, As with the different stereoisomers of 12, variation of the chiral centres in compound PZM21 results in large changes in efficacy and potency. Data are mean ± s.e.m. of normalized results (n = 3 measurements). b, Structure–activity relationship of compound 12 and 21 stereoisomers with affinities displayed as pKi values and agonist potency and efficacy in a Gi/o Glosensor assay. c, d, PZM21 docked to active μOR shows a more extended conformation as compared to the inactive state. e, In the docked active state, the PZM21 thiophene extends into the specificity-determining region of opioid receptors. Key interacting residues here are highlighted as red lines and corresponding residues at the other human opioid receptors are indicated. f, Docked pose of TRV130 within the μOR site, showing minimal overlap in key pharmacophores with PZM21 besides the ionic interaction between the cationic amine and D1473.32. g, Molecular dynamics simulations of PZM21 in the inactive μOR state (grey and black traces) leads to a stable conformation with the thiophene positioned > 10 Å away from N1272.63 (total of 2 μs of simulation time over three independent trajectories). In contrast, PZM21 adopts a more extended pose when simulated with active μOR, with an average distance of 6 Å between the thiophene and N1272.63. Other key interactions between μOR and PZM21 are also highlighted.
