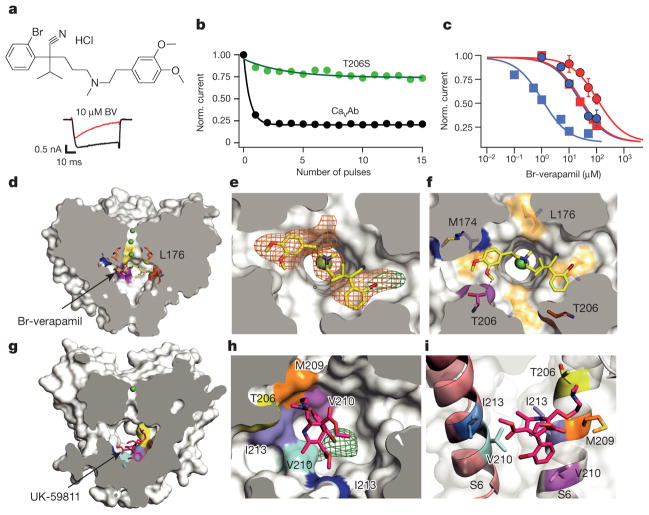
Figure 4. State-dependent inhibition by pore block with Br-verapamil and UK-59811.
a, Br-verapamil. Ba2+ current records for CaVAb with 0 μM (black) and 10 μM (red) during the depolarizing pulse. b, State-dependent block of CaVAb (n = 7) and CaVAb T206S (n = 3) at 10 μM during trains of depolarizations at 1 Hz from −120 mV to 0 mV. The error bars for all the data points on this graph are too small to be visible. c, Inhibition by Br-verapamil for CaVAb and CaVAb T206S at V = −120 mV and following trains of depolarizations as in b. CaVAb: resting state block, blue circles, IC50 = 24 ±1.6 μM; state-dependent block, blue squares, IC50 =810 ±80 nM. CaVAb T206S: resting state block, red circles, IC50 = 115±3.2μM; state-dependent block, red squares, IC50 =24±0.8μM; n =3–11; mean ± s.e.m. d, Side view of the pore module sectioned through the selectivity filter with Br-verapamil bound (yellow sticks). Ca2+, green spheres. e, Fo–Fc electron density (2.5σ, orange mesh) and anomalous scattering density (3σ, green mesh) for Br defines location of Br-verapamil. f, The two aromatic rings of verapamil are close to T206 of adjacent subunits. g, UK-59811 (red sticks) binds with its dihydropyridine ring deep in the central cavity. h, Anomalous scattering density (3.5σ, green mesh) of Br in UK-59811. i, S6 segments with residues surrounding UK-59811 in sticks.