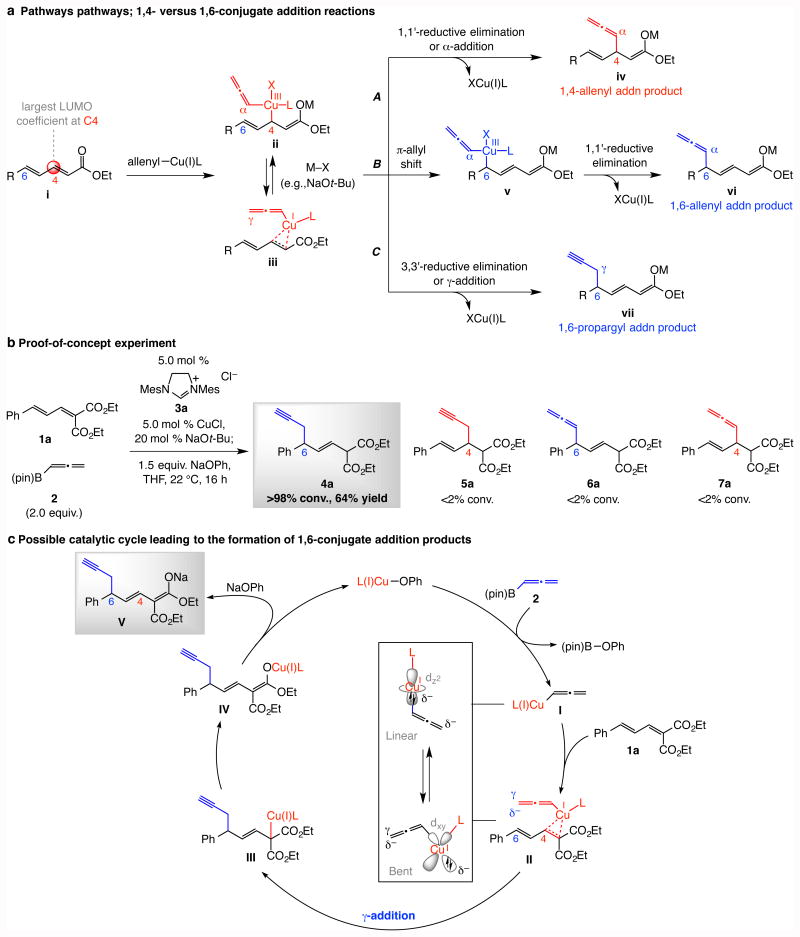
Figure 1. Possible conjugate addition pathways, the initial experiment and a plausible catalytic cycle.
a, In a conjugate reaction, addition to the C4 site is kinetically favored (→ii or iii); subsequent 1,1′-reductive elimination (or α-addition) could afford product iv (Route A), or a 1,3—π-allyl shift (→v) may precede reductive elimination, affording 1,6-allenyl addition product vi (Route B). Alternatively, ii/iii may be directly converted to vii by a γ-addition (3,3′-reductive elimination type) process (Route C). b, Proof-of-principle experiment indicates that with an allenyl–copper intermediate, Route C predominates. c, Plausible catalytic cycle for the preferential formation of the 1,6-propargyl addition product. Abbreviations: R, or G, various organic functional groups; LUMO, lowest unoccupied molecular orbital; M, metal; pin, pinacolato; Mes, 2,4,6-trimethylphenyl.