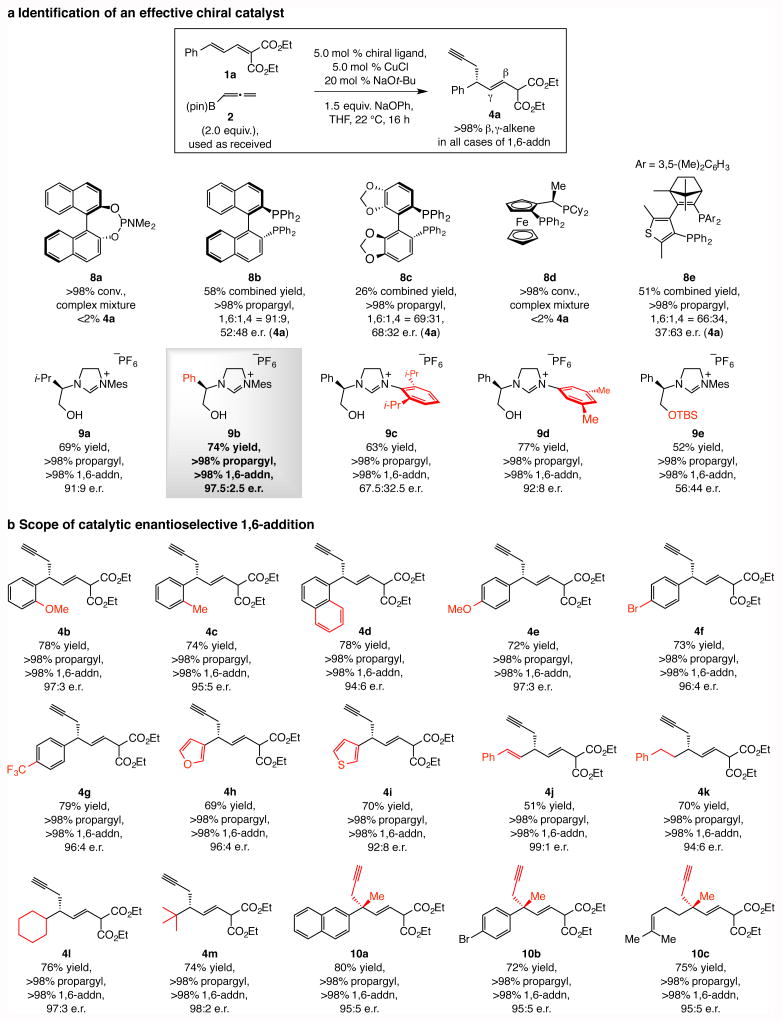
Figure 2. Catalytic enantioselective 1,6-propargyl conjugate additions.
a, Screening of a variety of chiral phosphine and NHC ligands indicated that the chiral copper catalysts derived from the latter series are significantly more effective, and that corresponding to imidazolinium salt 9b is optimal. b, The catalytic process is broadly applicable, affording products uniformly with >98% propargyl and 1,6-addition selectivity and in up to 80% yield and 98:2 e.r. Products containing a tertiary or an all-carbon quaternary carbon stereogenic center can be accessed. Abbreviations: pin, pinacolato; TBS, tert-butyldimethylsilyl, Mes, 2,4,6-trimethylphenyl; Mes, 2,4,6-trimethylphenyl.
Reactions were performed under N2 under the conditions shown in the box for 4a, except for 10a-c, where 10 mol % 9b and CuCl were used and mixture was allowed to stir for 24 h. Conversions, propargyl:allenyl and 1,6-:1,4-addition ratios were measured by analysis of 1H NMR spectra of unpurified mixtures; the variance of values estimated to be <±2%. Yields correspond to isolated and purified products and represent an average of at least three runs (±5%). See the Supplementary Information for experimental details and spectroscopic analyses.