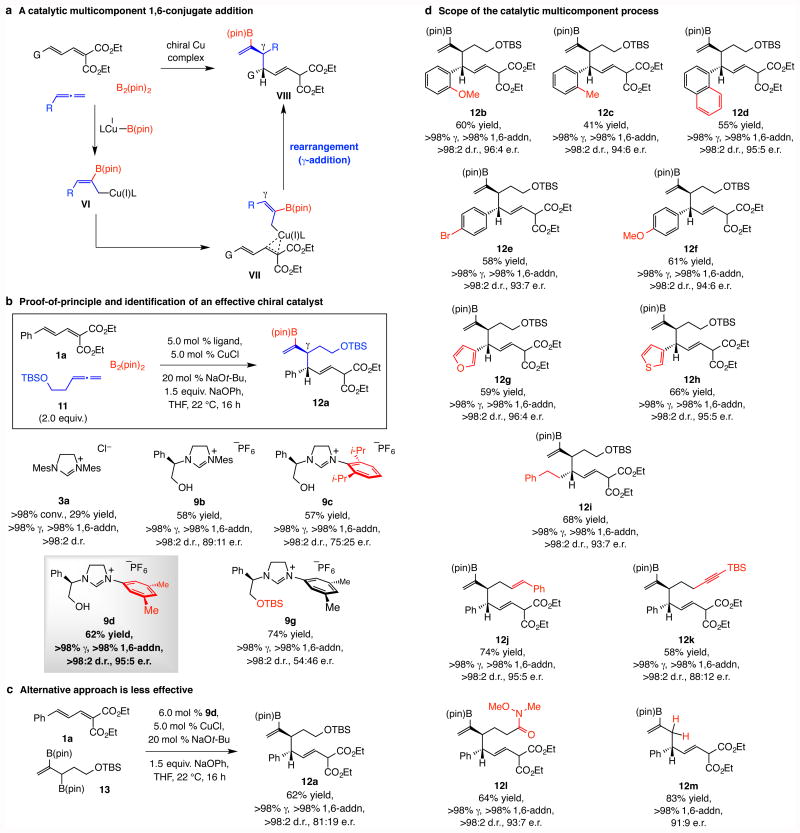
Figure 3. Catalytic diastereo- and enantioselective multicomponent 1,6-conjugate addition of 2-B(pin)-substituted allyl moieties.
a, The pathway through which 1,6-addition products may be generated by a multicomponent process involving a dienoate, an allene and B2(pin)2. b, Preliminary experiment with an achiral NHC–Cu complex demonstrates that, although inefficient, reactions are exceptionally γ-, group- and 1,6-selective. Screening studies to identify an effective chiral catalyst indicates that a different NHC ligand is optimal for these transformations (vs. propargyl additions). c, The alternative approach entailing initial synthesis of a diboryl reagent leads to lower enantioselectivity. d, The catalytic protocol has considerable scope. Abbreviations: pin, pinacolato; TBS, tert-butyldimethylsilyl.
Reactions were performed under N2 under the conditions shown for synthesis of rac-12a (Fig. 3b). Conversions, propargyl:allenyl, 1,6-:1,4-addition and diastereomeric ratios (d.r.) were measured by analysis of 1H NMR spectra of unpurified mixtures; the variance of values estimated to be <±2%. Yields correspond to isolated and purified products and represent an average of at least three runs (±5%). Ketone 12k was obtained after oxidative work-up. See the Supplementary Information for experimental details and spectroscopic analyses.