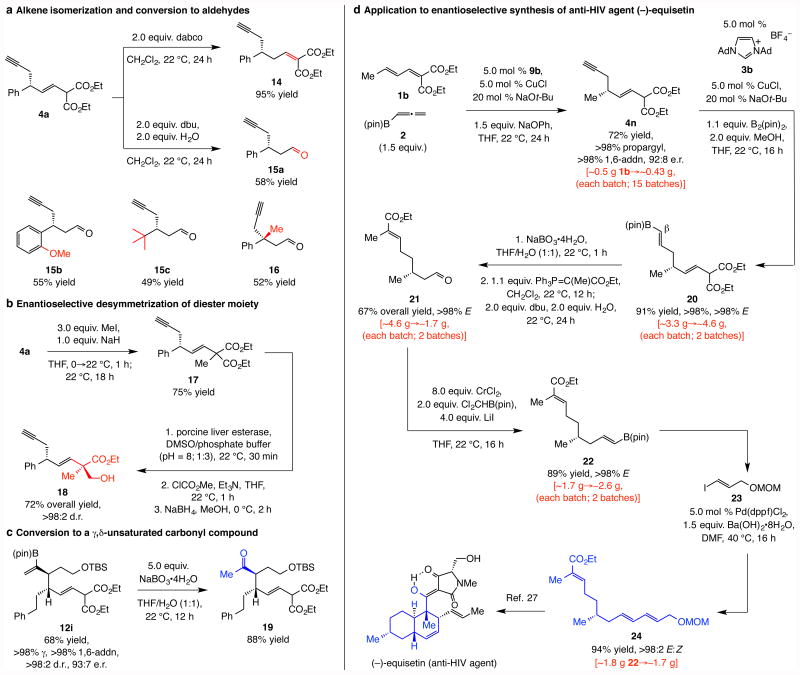
Figure 5. Functionalizations and demonstration of utility.
a, The kinetically favored alkene can be readily isomerized to the thermodynamically preferred isomer under one set of basic conditions, while with water present, cleavage of the diester moiety leads to the formation of β-substituted aldehydes. b, Alkylation followed by enzymatic desymmetrization of the diester unit proceeds with excellent stereochemical control. c, Oxidation of the alkenyl–B(pin) moiety affords otherwise difficult-to-access γ,δ-unsaturated ketones with vicinal stereogenic centers at the α- and β-carbon sites. d, Application to synthesis of gram quantities of enantiomerically enriched triene 24, previously used in the total synthesis of anti-HIV agent (–)-equisetin showcases utility of the catalytic approach. Abbreviations: dabco, 1,4-diazabicyclo[2.2.2]octane; dbu, 1,8-diazabicyclo[5.4.0]undec-7-ene ; DMSO, dimethylsulfoxide; Ad, adamantyl; dppf, 1,1′-bis(diphenylphosphino)ferrocene; MOM, methoxymethyl; pin, pinacolato.