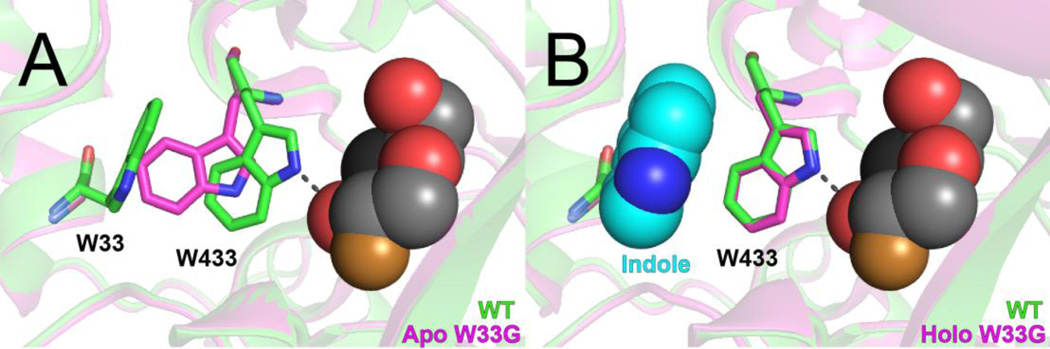
Figure 1. Structural basis for inactivation and rescue in β-gly W33G.
A comparison of the previously-determined crystal structures of wild type β-glycosidase (green) with its W33G mutant (pink). A covalent substrate analog (2-fluoro-2-deoxy-D-glucose, spheres) is included from WT structure to indicate the location of the active site; this compound was not present in either structure of β-gly W33G. (A) In the apo structure of β-gly W33G, W433 shifts into cavity that was previously occupied by W33; thus, W433 is no longer positioned to participate in hydrogen bonding with the substrate. (B) Upon soaking with indole, the holo structure of β-gly W33G shows that W433 has returned to its original position; indole (cyan spheres) occupies the cavity resulting from the W33G mutation.