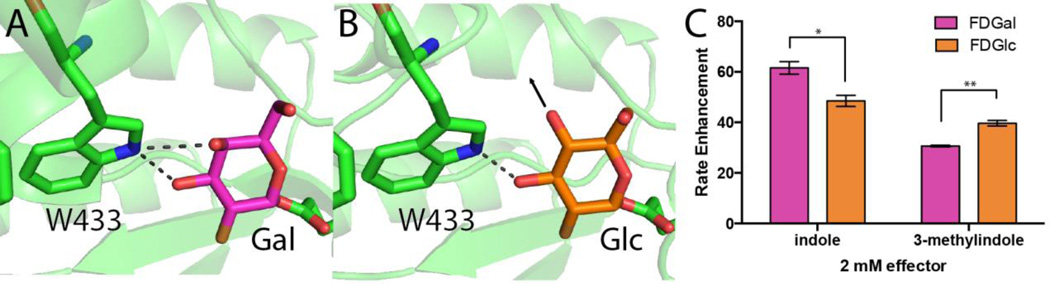
Figure 4. Trp433 forms different interactions with Gal versus Glc, allowing effectors to rescue to different extents, leading to slightly different effector preferences.
Crystal structures of wild type β-glycosidase bound to covalent substrate analogs (A) 2F-Gal and (B) 2F-Glc. The active site residue Trp433 makes distinct hydrogen bond interactions depending on the sugar’s stereochemistry at the C4 position. (C) Enhancement of initial velocity (relative to the apo enzyme) for substrates that differ only at this stereocenter (FDGal versus FDGlc), rescued using either indole or 3-methylindole. Assays were carried out using 250 μM substrate and 2 mM effector. Bars indicated have a statistically significant difference in their mean values (* with p < 0.05, ** with p < 0.01).