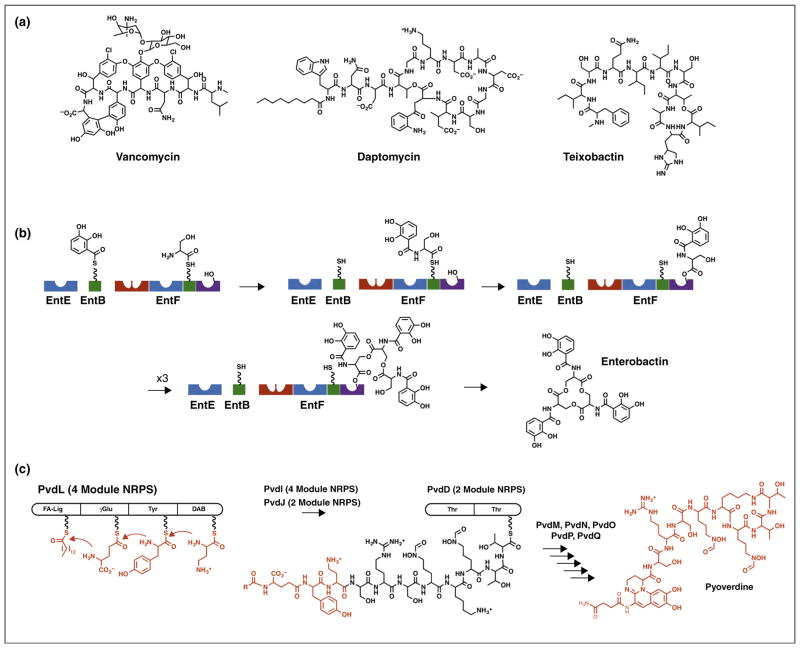
Figure 1.
Biosynthetic assembly lines of NRPS proteins. (a) NRPS products vancomycin, daptomycin, and teixobactin demonstrate the diversity of NRPS products. (b) Protein domains catalyze steps in the biosynthesis of the siderophore enterobactin. Blue adenylation domains load 2,3-dihydroxybenzoic acid (EntE) or serine (EntF) onto partner PCP domains (green) in intermolecular or intramolecular reactions. The red condensation domain of EntF then catalyzes the transfer of DHB from the loaded EntB carrier to form the amide with serine on EntF. This amide is then transferred to a catalytic serine within the (purple) thioesterase domain. After three cycles of synthesis, the thioesterase domain catalyzes release of the mature enterobactin molecule. The carrier domain of EntF must therefore sequentially visit the active sites of the adenylation, condensation, and thioesterase domains. (c) The pyoverdine biosynthetic pathway involves four NRPS proteins. The four-module PvdL protein initiates synthesis with the modules that direct incorporation of myristic (or myristoleic) acid, glutamic acid, tyrosine, and 2,4-diaminobutyrate. The tetrapeptide is passed to and extended by neighboring NRPSs PvdI and PvdJ, not shown, and ultimately to the two-module NRPS PvdD. The terminal thioesterase domain of PvdD releases the pyoverdine molecule through a cyclization step, where additional enzymes complete the maturation to produce pyoverdine.