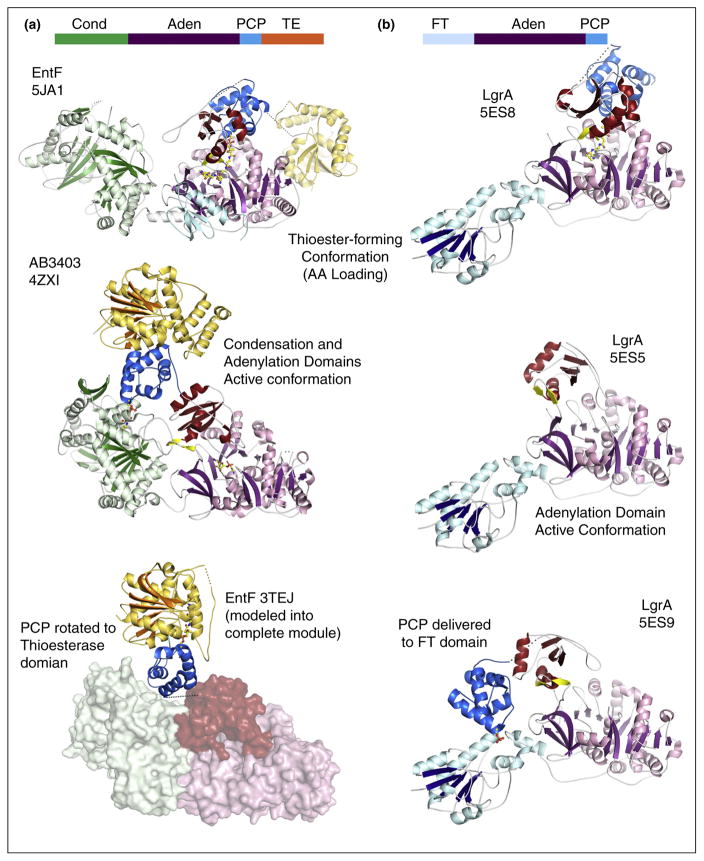
Figure 3.
Recent structures of multidomain NRPS proteins. Structures of (a) termination modules AB3403 and EntF and (b) LgrA in multiple conformations illustrate the dynamics used to deliver the PCP to multiple catalytic active sites. The top of each panel shows the domain architecture with condensation (Cond), adenylation (Aden), PCP, thioesterase (TE), and formyltransferase (FT) indicating the position of the domains within the protein. The terminal modules adopt the adenylation conformation (Panel a, middle) to carry out the initial adenylation of the amino acid substrate. Rotation of the C-terminal subdomain delivers the PCP to the adenylation domain for covalent loading (thiolation) step (Panel a, top). Return of the protein to the adenylate-forming conformation simultaneously delivers the PCP to the condensation domain (Panel a, middle) where the loaded PCP is positioned to receive the upstream substrate for peptide bond formation. Rotation of the PCP into the thioesterase domain (Panel a, bottom) would deliver the PCP here for removal of the loaded peptide. The bottom panel illustrates the functional complex of the PCP and thioesterase domain from EntF (PDB 3TEJ) superimposed onto the position of the thioesterase domain of AB3403. In the structural cycle of the LgrA protein, the same two initial steps, adenylation (Panel b, middle) and thioester forming (Panel b, top) are illustrated in crystal structures. The third state illustrates the delivery of the loaded PCP to the formyltransferase domain for modification of the loaded substrate (Panel b, bottom). The extension of the C-terminal subdomain (red) of the adenylation domain enables this functional interaction.