Figure 1.
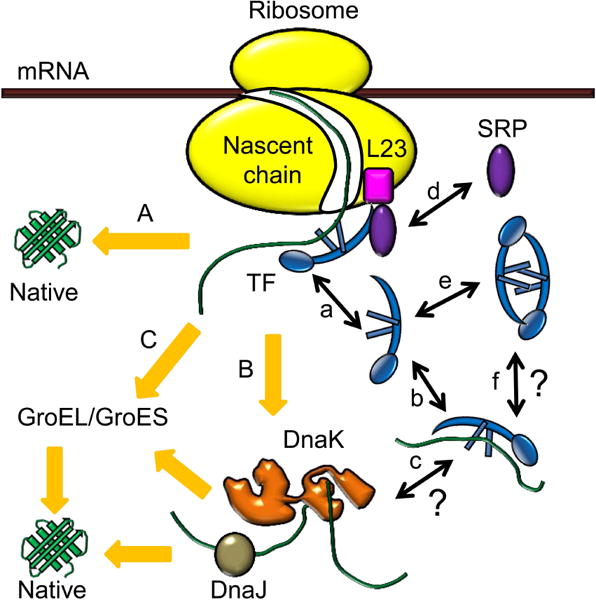
Molecular chaperone systems in E. coli, and functions of trigger factor (TF). Upon emerging from the ribosome, nascent polypeptides first interact with the ribosome-associated TF to fold to native states (pathway A). The polypeptides needing further assistance are transferred to the DnaK/DnaJ system (pathway B) or the GroEL/GroES system (pathway C) to complete folding. When associated with the ribosome, TF binds to the ribosomal protein L23 near the polypeptide exit site and goes through binding-unbinding cycles (a). In the cytoplasm, TF can bind to unfolded or partially folded polypeptides (b), but whether these TF-polypeptide complexes can interact with the DnaK/DnaJ system is unclear (c). Besides TF, another ribosomal associated factor, signal recognition particle (SRP), can also bind to L23 on the ribosome (d). The free TFs in the cytoplasm can exist as both monomers and dimers (e), but the potential role of TF2 dimer in binding to unfolded or partially folded polypeptides remains to be elucidated (f).
